Common variants in QPCT gene confer risk of schizophrenia in the Han Chinese population
Abstract
Schizophrenia (SCZ) is a common and severe mental disorder, its etiology has not been elucidated completely. In one previous genome-wide association study (GWAS) of SCZ in the Caucasian population, the QPCT has been reported as susceptible gene for SCZ. The QPCT gene encodes Glutaminyl cyclase (QC), an enzyme which is involved in the post translational modification by converting N-terminal glutamate of protein to pyroglutamate, which is resistant to protease degradation, more hydrophobic, and prone to aggregation and neurotoxic. To further investigate the role of this gene in the pathogenesis of schizophrenia in the Han Chinese population, we conducted this study in 1,248 (Mean age ± S.D, 36.44 years ± 9.0) SCZ cases, 1,248 (Mean age ± S.D, 30.62 years ± 11.35) healthy control samples for a case control study. We genotyped six SNPs in this study, including one positive SNP of the previous study, using the Sequenom MassARRAY platform. We found that rs2373000 was significantly associated with SCZ before correction [rs2373000: P allele = 0.016, χ2 = 5.784, OR [95%CI] = 0.861 [0.762–0.972], P genotype = 0.018, χ2 = 0.069]. After permutation correction for multiple testing, rs2373000 [rs2373000: P Allele corrected = 0.063, P genotype corrected = 0.069] showed marginal association with SCZ. Additionally, one pathogenic haplotype (TGT) containing rs2373000 was also significantly associated with SCZ. Our results are consistent with the findings of previous study and the genetic risk of QPCT gene for SCZ also exists in the Han Chinese population. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Schizophrenia (SCZ) is a common and severe psychiatric disorder. The population prevalence of SCZ is 1% and heritability is 70–85 % [Burmeister et al., 2008]. It is also evident from family, adoption, and twin studies that heritability in the development of SCZ is 64% [Paul et al., 2009]. Disability-adjusted life years (DALYs) are 7.4% for Schizophrenia [Whiteford et al., 2013]. The exact etiology of SCZ is unknown, however based on the family, adoption, and twin studies, we may conclude that genetic factors play an important role in the development of this disease [Craddock, 2005]. Nowadays, molecular approaches like candidate gene association study and GWAS (genome wide association study) have proposed various susceptible genes which play an important role in the pathogenesis of SCZ. Meanwhile, QPCT gene has been reported as a new locus for SCZ in Caucasian by GWAS plus meta-analysis and replication study [Ripke et al., 2013].
QPCT gene is located on chromosome 2 at co-ordinate 37571753-37600465, spanning about 28.71–Kbp of DNA and contains seven exons (NM_012413) (http://genome.ucsc.edu/cgi-bin/hgGateway). This gene encodes Glutaminyl cyclase (QC), an enzyme which shows high level of expression in brain and other peripheral tissues. This enzyme catalyzes the formation of pyroglutamate (pGlu) at the N terminus of the various peptides and proteins [Sykes et al., 1999; Hartlage-Rübsamen et al., 2009]. The QC is localized in the Golgi complex, endoplasmic reticulum, and secretary granules where it is supposed to play an important role in maturation of different proteins [Cynis et al., 2008a; Hartlage-Rübsamen et al., 2009].
Glutaninyl cyclase (QC) enzyme has also been reported to involve in post translational modification of amyloid- β peptide by converting N terminal glutamate of this peptide at position 3 or 11 into pyroglutamic acid, which is resistant to protease degradation, more hydrophobic and prone to aggregation which is neurotoxic [Cynis et al., 2008b; Kimpe et al., 2012].
Currently, it is known that unsettled Ca2+ homeostasis not only enhances mRNA level of QC but also its enzyme activity. The QC promoter is considered to contain binding sites for calcium dependent transcription factors such as c-fos and c-jun. These two transcription factors are proposed to be induced by the same Ca2+ related stimuli as QC, and their upregulation precedes QC expression. This mutual up regulation of enzyme and these factors is not observed in non-neuronal cell. Upregulation of QC selectively in neuronal cells via Ca2+ dependent transcription factors is the major consequence of unsettled Ca2+ homeostasis [Kimpe et al., 2012].
Previous GWAS of schizophrenia in the Caucasian with meta-analysis and replication has shown significant association of single nucleotide polymorphism (SNP), rs2373000 (P = 6.78 × 10−9) with SCZ [Ripke et al., 2013]. In our study, a case control analysis was designed to further investigate whether common variants in this gene are associated with schizophrenia in the Han Chinese population. Total six SNPs including one positive SNP of previous study covering this gene were included for genotyping by using Sequenom MassARRAY platform.
MATERIALS AND METHODS
Samples/Subjects
Totally 1,248 unrelated SCZ patients (845 males and 403 females) and 1,248 healthy controls (672 males and 576 females) were included in this study. The mean ages of SCZ patients and healthy controls were 36.44 years (±9.0) and 30.62 years (±11.35), respectively.
All the patients were out-patients or sometimes stable in-patients, Shanghai in origin and living in Shanghai China. Patients were interviewed by two independent psychiatrists and diagnosed strictly according to DSM-IV criteria (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) based on SCID-1 (structured Clinical Interview for DSM-IV Axis 1 Disorders). Normal controls were selected from general public of the Han Chinese Population in Shanghai. The control subjects were also interviewed by two independent psychiatrists according to DSM-IV criteria based on (SCID-1) [American Psychiatric Association, 1994].
In written information from volunteers regarding their medical histories with detailed questions about psychosis, and other complex disease was obtained. A face to face interview was conducted from volunteers before blood collection and physical examinations such as height, weight, blood pressure, etc. were also carried out.
Informed consent was obtained from all the subjects and the study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). This study was reviewed and approved by the local ethical committee.
SNP Selection and Genotyping
Genomic DNA was extracted from peripheral blood samples using Quick Gene DNA whole blood kit L (FUJIFILM) protocol. Five tag SNPs (rs6708524, rs3770752, rs4384764, rs3770748, rs6708310) were selected using haploview software version 4.2, with pair-wise tagging r2 ≥ 0.6 and minor allele frequency (MAF) ≥0.05 [Barrett et al., 2005] and human genome browser of the University of California-Santa Cruz (UCSC) (http://genome.ucsc.edu/). In addition to this, one positive SNP of previous study (rs2373000) was also included in this study. Detail of six SNPS is given in the Table I. Relative position of SNPs obtained from Vector NTI (www.invitrogen.com/VectorNTI) is shown in (Fig. 1). The coverage of tag SNPs for QPCT gene was calculated by using Haploview, which was 100% [Barrett et al., 2005]. All the selected SNPs were genotyped using the Sequenom MassARRAY matrix—assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry platform (Sequenom, San Diego, CA) using DNA isolated from peripheral leucocytes of blood samples.
| SNP ID | rs6708524 | rs3770752 | rs4384764 | rs2373000 | rs3770748 | rs6708310 |
|---|---|---|---|---|---|---|
| Position | Chr. 2:37572687 | Chr. 2:37576136 | Chr. 2:37590284 | Chr. 2:37592628 | Chr. 2:37595525 | Chr. 2:37600427 |
| Functional | Intron | Intron | Intron | Intron | Intron | 3-UTR |
| Polymorphism | A/G | C/T | A/G | C/T | C/T | A/G |
MassARRAY design software package (v4.0) was used to design the specific SNP filtering. The quality of PCR amplified fragments and extension primer specificity was ascertained prior to running the reaction. Shrimp Alkaline phosphate phosphatase was utilized for the dephosphorylation of residual nucleotides prior to iPLEX Gold reaction. Following a single-base extension, reaction products were desalted with spectro clean resin (Sequenom), and 10 nl was spotted onto the SpectroCHIP using the MassARRAY Nanodispenser.
The MassARRAY Analyzer Compact MALDI-TOF mass spectrometer was used to determine product mass. In order to clarify uncertain genotype calls, a manual review was done. An assay with a call rate of less than 80% within the same Spectro Chip was considered to have failed. The overall call rate in our assay was more than 97%.
Statistical Analysis
Allele and genotype frequencies, haplotype analysis, Hardy-Weinberg equilibrium (HWE) analysis, 1,000 permutation tests, and association tests for schizophrenia were performed by using online SHEsis software (http://shesisplus.bio-x.cn/SHEsis.html) [SHI and HE, 2005; Li et al., 2009]. This is a user friendly software platform which is equipped with a series of highly efficient analytic tools designed for association studies.
The Genetic Power Calculator (http://pngu.mgh.harvard.edu/∼purcell/gpc/) was used to calculate case-control genetic power [Purcell et al., 2003]. The statistical significance was assumed at the threshold of 0.05. A total 1,000 permutation were carried out to correct the P values for multiple testing. The χ2 test was used to evaluate Hardy–Weinberg equilibrium. Differences in schizophrenia cases and controls were also evaluated by using χ2 test. The D'value between markers was used to represent the linkage disequilibrium. The only those haplotypes with an estimated frequency >3% were included for analysis.
RESULTS
Single Site Analysis
The P values of Hardy–Weinberg equilibrium (HWE) for one SNP exceeded from 0.05 in healthy controls. In the following studies, we excluded SNP (rs6708524) due to its failure in Hardy–Weinberg equilibrium tests (P < 0.05).
The allele and genotype frequencies of five SNPs in the patient samples and normal controls were shown in Table II. For SCZ, rs2373000 showed significant association before correction [rs2373000: P allele = 0.016, χ2 = 5.784, OR (95%CI) = 0.861 (0.762–0.972), P genotype = 0.018, χ2 = 8.001]. After 1,000 permutation correction, rs2373000 showed marginal association with SCZ [rs2373000: P corrected allele = 0.063, P corrected genotype = 0.069] (Table II). It is important to note that the same risk allele (T) for corresponding positive SNP in previous [Ripke et al., 2013] as well as in our study is same which further supports our results (Table II).
| SNP | Alleles | OR [95%CI] | χ2 | P-Value | Permutation P | Genotypes | χ2 | P-Value | Permutation P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs3770752 | T | C | T/T | T/C | C/C | |||||||
| SCZ | 2218(0.895) | 258(0.104) | 0.859 [0.718–1.027] | 2.754 | 0.096 | 0.335 | 999 (0.806) | 220 (0.177) | 19 (0.015) | 3.022 | 0.22 | 0.618 |
| Control | 2091(0.88) | 283(0.119) | 924 (0.778) | 243 (0.204) | 20 (0.016) | |||||||
| rs4384764 | G | A | G/G | G/A | A/A | |||||||
| SCZ | 1461(0.591) | 1007(0.408) | 1.004 [0.896–1.126] | 0.006 | 0.935 | 1 | 431 (0.349) | 599 (0.485) | 204 (0.165) | 0.034 | 0.982 | 1 |
| Control | 1414(0.593) | 970(0.406) | 416 (0.348) | 582 (0.488) | 194 (0.162) | |||||||
| rs2373000 | T | C | T/T | T/C | C/C | |||||||
| SCZ | 1786(0.719) | 696(0.28) | 0.861 [0.762–0.972] | 5.784 | 0.016 | 0.063 | 651 (0.524) | 484 (0.39) | 106 (0.085) | 8.001 | 0.018 | 0.069 |
| Control | 1717(0.688) | 777(0.311) | 584 (0.468) | 549 (0.44) | 114 (0.091) | |||||||
| rs3770748 | T | C | C/C | T/C | T/T | |||||||
| SCZ | 1250(0.507) | 1214(0.492) | 0.982 [0.877–1.099] | 0.095 | 0.756 | 0.997 | 299 (0.242) | 616 (0.5) | 317 (0.257) | 0.096 | 0.953 | 1 |
| Control | 1219(0.511) | 1163(0.488) | 284 (0.238) | 595 (0.499) | 312 (0.261) | |||||||
| rs6708310 | A | G | A/A | G/G | G/A | |||||||
| SCZ | 1389(0.575) | 1023(0.424) | 0.953 [0.85–1.069] | 0.648 | 0.42 | 0.857 | 405 (0.335) | 222 (0.184) | 579 (0.48) | 0.663 | 0.717 | 0.993 |
| Control | 1325(0.564) | 1023(0.435) | 377 (0.321) | 226 (0.192) | 571 (0.486) | |||||||
- Bold digits represent P < 0.05.
- SCZ, schizophrenia; CI, confidence interval; OR, odd ratio; χ2, chi square test.
In summary, after 1,000-permutation correction, rs2373000 marginally associated with SCZ [rs2373000: P corrected allele = 0.063, P corrected genotype = 0.069] (Table II). Some of the selected SNPs in the present study are in relatively strong linkage disequilibrium. Although the P-value after correction did not reach the global significant, this is the potential casual SNP because the global significant threshold is too stringent. However, our results are consistent with results of previous study and same risk allele is shared between these two studies. So, we successfully replicated rs2373000 in the Han Chinese population (Table II).
Linkage Disequilibrium
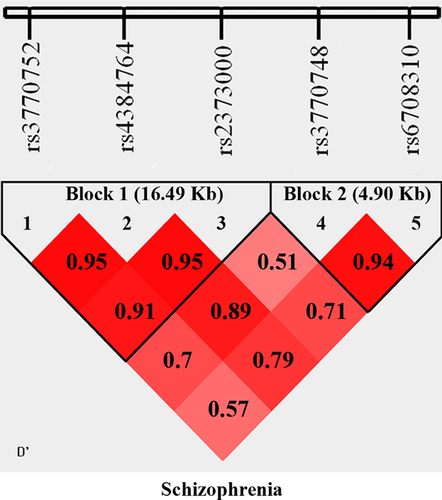
Adjacent SNPs with pairwise D’ ≥ 0.90 were classified in the same block. Therefore, two haplotype blocks were identified in SCZ. Haplotype blocks with the following sequence were analyzed in SCZ: rs3770752-rs4384764-rs2373000 (16.49 Kb) in block 1 and rs3770748-rs6708310 (4.90 Kb) in block 2 as shown in Figure 2.
Haplotype Analysis
Haplotype analysis results are shown in (Table III). One haplotype (TGT: P = 4.50 × 10−4, P corrected = 0.001) for the block rs3770752-rs4384764-rs2373000 showed significant association with SCZ. Notably, this haplotype contains corresponding positive SNP which was significantly associated in previous as well as in our study. Interestingly, positive haplotype containing risk allele (T) for corresponding positive SNP in our study and previous study is same (Table III).
| SNPs blocks with D′≥0.90 | Haplotype | Case (freq) | Control (freq) | χ2 | OR [95%CI] | P-value | Corrected P |
|---|---|---|---|---|---|---|---|
| rs3770752- -rs4384764- -rs2373000 | TGT | 769 (0.312) | 657 (0.277) | 12.314 | 1.246 [1.102–1.409] | 4.50e-04 | 0.001 |
| TGC | 438 (0.177) | 470 (0.198) | 1.378 | 0.917 [0.794–1.059] | 0.24 | ||
| TAT | 990 (0.401) | 946 (0.399) | 1.633 | 1.077 [0.961–1.207] | 0.201 | ||
| CGC | 242 (0.098) | 264 (0.111) | 1.064 | 0.907 [0.755–1.091] | 0.302 | ||
| rs3770748- -rs6708310 | CA | 1160 (0.482) | 1115 (0.475) | 1.635 | 1.075 [0.962–1.202] | 0.2 | |
| TA | 227 (0.094) | 209 (0.089) | 0.814 | 1.094 [0.899–1.332] | 0.366 | ||
| TG | 996 (0.413) | 990 (0.421) | 0.03 | 1.01 [0.901–1.131] | 0.862 |
- Haplotypes with frequency <0.03 in cases or controls were not included.
- Bold letters represent significant P value <0.05.
DISCUSSION
Schizophrenia is a severe psychiatric disorder. The complete etiology of this disorder is still unknown. The genetic factors play an important role in the pathogenesis of this disorder [Craddock, 2005]. Recently, Schizophrenia working group of the psychiatric genomic consortium (PGC) provided new insights regarding SCZ. In this study, schizophrenia related associations were profoundly enriched at enhancers active in brain rather than other tissues [Consortium, 2014].
Glutaminyl cyclase (QC) and its isoenzyme (iso QC) have been reported to modify N-terminal of CCL2, a chemokine which is associated with tumor progression in several cancer types. Expression of QPCT gene has been reported to correlate with mRNA level of substrate CCL2 in NF-kB dependent pathway [Kehlen et al., 2012]. Previous GWAS of schizophrenia in Caucasian, meta-analysis, and replication has shown significant association of rs2373000 (P = 6.78 × 10−9) in QPCT gene with SCZ [Ripke et al., 2013].
In our study, after permutation correction, rs2373000 [rs2373000: P corrected allele = 0.063, P corrected genotype = 0.069] showed marginal association with SCZ in the Han Chinese population. In previous study, minor allele (T) was more prevalent in schizophrenia cases than controls in Caucasian population. Similarly, in our study major allele (T) was also more frequently observed in schizophrenia cases than controls. The minor allele frequency in the Han Chinese population is (MAF = 0.31), whereas in the Caucasian population it is (MAF = 0.44). In the Caucasian population, the minor allele is (T) whereas in the Han Chinese population it is major allele. Notably, in both studies (T) allele emerged as the risk allele which hints that this marker may influence the activity of QPCT gene in schizophrenia.
Genetic power was calculated for rs2373000 in both allelic (1df) and genotypic (2df) models. For allele, power was 0.55 whereas for genotype it was merely 0.47. The genetic power calculation software is available at (http://pngu.mgh.harvard.edu/∼purcell/gpc/)[Purcell et al., 2003]. The genetic power analysis for aforementioned SNP indicates that we might find significant association of this SNP with SCZ if we increase the sample size while the genetic power is more than 0.8 in future studies. Comparing our study with previous one [Ripke et al., 2013], which has comparatively very large sample size. This is the first study, where we replicated the SNP (rs2373000) in the Han Chinese population.
Furthermore, one haplotype (TGT) for block rs3770752-rs4384764-rs2373000 containing corresponding positive SNP in our study was also significantly associated with SCZ [P corrected = 0.001], further validating this study.
Previous study conducted in the Chinese population, rs3770748 located in the intron of QPCT was found significantly associated with bone mineral density (BMD) in pre-menopausal (P = 0.002) and post-menopausal (P = 0.023) women [Huang and Kung, 2007]. Another study conducted in Japanese population has highlighted QPCT as the essential modifier of pituitary hormone, and single nucleotide polymorphism (SNP) variation inside this gene was attributed to BMD among postmenopausal women [Ezura et al., 2004]. In a multistage GWAS in the Caucasian, SNP (rs3770745) (P = 2.71 × 10−10) was successfully associated with Chronic Lymphocytic Leukemia [Berndt et al., 2013].
In summary, based on our results and potential involvement of this gene in different disorders, we may conclude that QPCT is an important gene which might regulate a key pathway toward pathogenesis of schizophrenia and other disorders. This is the first study which revealed that genetic risk exists in QPCT gene for Schizophrenia in the Han Chinese population. Comparing our study with previous one, previous study has comparatively very large sample size which is the limitation of our study. Further studies are needed to validate the association of this SNP with large sample size in the Chinese as well as other populations. It is also required to study the potential role of this gene in the correlated pathways.
ACKNOWLEDGMENTS
We thank and acknowledge all the participants of this study. This work was supported by the 973 Program (2015CB559100), the National 863 projects (2012AA02A515, 2012AA021802), the Natural Science Foundation of China (31325014, 81130022, 81272302, 81121001, 81171271), Shanghai Key Laboratory of Psychotic Disorders (13dz2260500), the Youth Research Project of Shanghai Health and Family Planning Commission (20134Y082), “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (12SG17), Program of Shanghai Subject Chief Scientist (15XD1502200), and the Shanghai Jiao Tong University Liberal Arts and Sciences Cross-Disciplinary Project (13JCRZ02).




