Support of association between BRD1 and both schizophrenia and bipolar affective disorder†‡
Mette Nyegaard and Jacob E. Severinsen contributed equally to this work.
How to Cite this Article: Nyegaard M, Severinsen JE, Als TD, Hedemand A, Straarup S, Nordentoft M, McQuillin A, Bass N, Lawrence J, Thirumalai S, Pereira ACP, Kandaswamy R, Lydall GJ, Sklar P, Scolnick E, Purcell S, Curtis D, Gurling HMD, Mortensen PB, Mors O, Børglum AD. 2009. Support of Association Between BRD1 and Both Schizophrenia and Bipolar Affective Disorder. Am J Med Genet Part B 153B:582–591.
Abstract
A recent study published by our group implicated the bromodomain containing protein 1 (BRD1) gene located at chromosome 22q13.33 with schizophrenia (SZ) and bipolar affective disorder (BPD) susceptibility and provided evidence suggesting a possible role for BRD1 in neurodevelopment. The present study reports an association analysis of BRD1 and the neighboring gene ZBED4 using a Caucasian case–control sample from Denmark and England (UK/DK sample: 490 patients with BPD, 527 patients with SZ, and 601 control individuals), and genotypes obtained from a BPD genome wide association (GWA) study of an overlapping English sample comprising 506 patients with BPD and 510 control individuals (UCL sample). In the UK/DK sample we genotyped 11 SNPs in the BRD1 region, of which six showed association with SZ (minimal single marker P-values of 0.0014), including two SNPs that previously showed association in a Scottish population [Severinsen et al. (2006); Mol Psychiatry 11(12): 1126–1138]. Haplotype analysis revealed specific risk as well as protective haplotypes with a minimal P-value of 0.0027. None of the 11 SNPs showed association with BPD. However, analyzing seven BRD1 SNPs obtained from the BPD GWA study, positive associations with BPD was observed with all markers (minimal P-value of 0.0014). The associations reported add further support for the implication of BRD1 with SZ and BPD susceptibility. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Family-, twin-, and adoptions studies have evidenced a substantial genetic component in the etiology of bipolar affective disorder (BPD) as well as in schizophrenia (SZ) [Cardno and Gottesman, 2000; Smoller and Finn, 2003]. An overlap in the symptomatology of both disorders is well known, and shared genetic susceptibility is likely according to epidemiological and linkage studies [Berrettini, 2003; Potash et al., 2003]. Several studies have implicated the telomeric region of chromosome 22q with SZ and BPD. Evidence of linkage or suggestive linkage has been reported in SZ samples, and positive LOD scores have likewise been reported in studies of BPD [Coon et al., 1994; Stober et al., 2000; Kelsoe et al., 2001; Mowry et al., 2004; Takahashi et al., 2005].
Analyzing a Faroese sample consisting of distantly related patients with BPD and SZ our group has previously reported that chromosome 22q13 may harbor two susceptibility loci shared for BPD and SZ (a 3.6 cM segment between D22S272 and D22S1140, and a >7 cM region telomeric to D22S1170) [Jorgensen et al., 2002]. In continuation of this we genotyped a case–control sample from Scotland for variations in the BRD1 gene (located in the 7 cM telomeric region) and found positive association with both SZ and BPD [Severinsen et al., 2006]. In the present study we performed a replication analysis of the reported association with BRD1 using a Caucasian case–control sample (the UK/DK sample) comprising patients and control individuals from England and Denmark (490 patients with BPD, 527 patients with SZ, and 601 control individuals) (Table I). In addition we analyzed all BRD1 SNPs found on the Affymetrix 500K array in an overlapping English BPD sample (UCL sample) (506 patients with BPD and 510 control individuals) that was genotyped as part of a large genome wide association study (GWAS) of BPD [Sklar et al., 2008].
| Samples | First reported in | Number of individuals | Genotyping platform | Significant association | ||
|---|---|---|---|---|---|---|
| Bipolar | Controls | SZ | ||||
| Scottish |
Severinsen et al. 2006 |
162 | 200 | 103 | SBE | SZ + BPD |
| UK/DK (Danish + English) | 490(165+325a) | 601(286+315a) | 527(248+279) | Sequenom | SZ | |
| WTCCC |
Wellcome Trust Case–Control Consortium 2007 |
1,864 | 14,311 | Affymetrix 500K | BPD | |
| UCL |
Sklar et al. 2008 |
506 | 510 | Affymetrix 500K | BPD | |
- WTCCC, Wellcome Trust Case–Control Consortium; SBE, single basepair extension using Applied Biosystem (Foster City, CA); SZ, schizophrenia.
- a These samples (325 BPD patients and 315 controls) were present in both the UCL sample and the UK/DK sample.
MATERIALS AND METHODS
Subjects
Two partly overlapping case–control samples were analyzed: The UK/DK sample and the UCL bipolar sample. The UK/DK sample included 490 patients with BPD, 527 patients with SZ, and 601 control subjects. The sample was made by merging a Danish sample (165 BPD, 248 SZ, 286 controls) and a sample from England (325 BPD, 279 SZ, and 315 controls) (Table I). All patients and control individuals were of Caucasian descent. The English BPD cases and control subjects included in the UK/DK sample were a subsample of the UCL bipolar sample comprising 506 BPD cases and 510 controls. The UCL sample was recently genotyped as part of a genome wide associations (GWA) study for BPD [Sklar et al., 2008]. There was no overlap between the present samples and the Scottish sample analyzed in our initial study [Severinsen et al., 2006].
The Danish patients were interviewed using the semi-structured diagnostic interview SCAN version 2.1. [Wing et al., 1998], and lifetime best estimate diagnoses according to the ICD-10 classification of mental and behavioral disorders, diagnostic criteria for research (ICD-10-DCR) [WHO, 1993] and DSM-IV [American Psychiatric Association, 2000] were made by two psychiatrists. All BPD cases met the ICD-10-DCR criteria for BPD and DSM-IV criteria for bipolar I disorder. The individuals suffering from SZ were fulfilling DSM-IV and ICD-10-DCR criteria for SZ. The Danish control subjects consisted of ethnically matched (Danish Caucasians) unscreened controls, which were blood donors from the National Blood Service in Odense, Funen and university students from Aarhus.
The English patients were interviewed using the SZ and Affective Disorders Schedule—Life Time version (SADS-L) and diagnosed according to ICD-10-DCR, Research Diagnostic Criteria (RDC), and DSM-III-R [Spitzer and Endicott, 1977; Spitzer et al., 1978]. All BPD cases met the ICD-10-DCR criteria for BPD and DSM-III-R criteria for bipolar I disorder. The individuals suffering from SZ fulfilled DSM-IV and ICD-10-DCR criteria for SZ. The English control subjects were recruited from London branches of the National Blood Service, from local NHS family doctor clinics and from university student volunteers. All control subjects were interviewed with the SADS-L to exclude all psychiatric disorders including alcohol dependence according to RDC/DSM-III-R criteria as well as drinking above the upper limit for safe drinking of 21 units per week for males and 14 units for females as defined by the Royal College of Psychiatrists. The control subjects were further selected on the basis of not having a family history of BPD, SZ, or alcoholism.
All subjects gave informed consent prior to inclusion, and study approval was obtained from the local research ethical committees.
Genotyping
Genomic DNA was isolated from blood samples according to standard procedures for all samples in this study. The SNPs selected for genotyping in the UK/DK sample included all the five BRD1 SNPs analyzed in the initial association study of Scottish samples [Severinsen et al., 2006]. In addition, three BRD1 SNPs and four SNPs in the neighboring ZBED4 gene were included. Of the 12 SNPs selected, 1, rs138881, was excluded due to failing PCR in all samples.
Genotyping in the UK/DK sample was carried out using the Sequenom MassARRAY Genotyping system (Sequenom, San Diego, CA). Primers for PCR and extension probes were designed using the MassARRAY Assay Design 3.1 software (Sequenom). Multiplex PCR was performed in 5 µl reactions containing 10 ng of genomic DNA, 1.25× PCR buffer (Qiagen, Valencia, CA), 0.5 mM dNTP (Roche, Geneva, Switzerland), 100 nM of each primer (Metabion, Martinsried, Germany), and 0.5 U Taq polymerase (Qiagen) and using standard cycling conditions described by Sequenom. The PCR products were treated with SAP and the probe extension reaction (iPLEX) was carried according to the iPLEX standard protocol (Sequenom). The iPLEX reactions were desalted using resin and spotted on a SpectroCHIP (Sequenom) using a nanodispenser. The samples were analyzed using a Bruker matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometer (Sequenom) and the genotypes were determined using the MassARRAY Typer 3.4 software (Sequenom). Assay conditions and primer sequences are available upon request.
Genotypes of 36 SNPs located within other genes, also typed in the UK/DK sample using the Sequenom platform, were used to evaluate possible population substructure (stratification). rs numbers of these SNPs are available upon request. To estimate the genotyping error rate, 200 samples were genotyped in duplicates. The genotyping error rate was across all SNPs below 0.005. In particular, we found zero conflicts for rs138820, the strongest associated marker in our study.
For the UCL sample, genotyping was performed at the Broad Institute of Harvard and MIT using the Affymetrix 500K array [Sklar et al., 2008]. Genotypes from all SNPs within the BRD1 and ZBED4 gene located on the Affymetrix 500K chip were drawn from the large dataset and analyzed for the present study. Genotypes from the BRD1 gene in the UCL sample have not previously been published.
Samples Included for Meta-Analysis
For a meta-analysis of Sequenom SNP we included data from the present study (UK/DK sample) and from our initial association a study in a Scottish case–control sample [Severinsen et al., 2006].
For a meta-analysis of Affymetrix SNPs, we included data from the present study (UCL bipolar sample) and from the publicly available case–control study of BPD performed by the Wellcome Trust Case–Control Consortium (WTCCC) [Wellcome Trust Case–Control Consortium, 2007]. From the WTCCC website we downloaded allele frequencies and P-values for association with BPD (comparing patients with BPD with combined controls) for SNPs in the BRD1 and ZBED4 gene (http://www.wtccc.org.uk).
Imputing
In the UK/DK sample (n = 1,621) genomic coverage was increased by imputing genotypes for 25 additional SNPs from six Sequenom SNPs (rs138820, rs138841, rs138864, rs138880, rs910799, rs4624) using MACH1 [Li and Abecasis, 2006]. Imputation was performed for the region 48,484,939–48,602,778 (Build35 Jan07 version) and using haplotypes from the CEU population from Phase I of the HapMap project (http://www.hapmap.org/cgi-perl/gbrowse/hapmap_B35/). The remaining five Sequenom markers (out of 11 Sequenom markers in total) did not provide information for imputation, as they were not genotyped during HapMap phase I. A quality threshold for the imputed SNPs was applied so that only SNPs predicted with a likelihood of 0.9 was used for further analysis (quality >0.90 in the info file from the MACH1 software).
Statistical Analysis
Data quality control and association analysis were performed using the open-source toolset PLINK [Purcell et al., 2007] (http://pngu.mgh.harvard.edu/purcell/plink/). Single marker association analysis was performed using the Cochran–Armitage trend test. To refine the signal in the UK/DK sample, haplotype association was performed using a sliding window approach of two- and three-marker haplotypes. Nominal P-values <0.05 are referred to as significant.
Correction for multiple testing was performed using the method proposed by Nyholt http://genepi.qimr.edu.au/general/daleN/SNPSpD [Nyholt, 2004]. In this method, the effective number of independent tests (VeffLi) was calculated from each set of SNPs by spectral decomposition of a matrix of pairwise linkage disequilibrium (LD) values (r-values) and an experiment-wide significance threshold required to keep type I error rate at 5% was calculated as 0.05/VeffLi.
The degree of LD in the CEU population and in the UK/DK sample was calculated with the program HaploView [Barrett et al., 2005].
Tests for genetic homogeneity across samples were performed by applying an exact test as implemented in Arlequin [Excoffier et al., 2005] using nine SNPs. P-values for significance was obtained using 10,000 steps of Markov Chain and 10,000 dememorization steps. These nine SNPs are all located on chromosome 22, but were chosen among a set of 36 genotyped SNPs, all from outside the region of interest, in such a way that they were not in strong pairwise LD (D′ < 0.3 and r2 < 0.1) and can therefore be considered independent. To evaluate the minimum detectable level of genetic differentiation, the power of the exact test was simulated for different levels of genetic differentiation measured as Fst (range 0–0.5). Power simulations were performed as implemented in PowSim [Ryman and Palm, 2006] by repeating t generations of drift 200 times for each value of Fst (different values of Fst were obtained by varying t). For each locus a burnin of 1,000 and a total of 100,000 iterations were performed. An effective population size of Ne = 3,000 was assumed, and sample sizes were as in the present study.
Whether any of the 36 markers showed increased genetic differentiation (Fst) between samples compared to the other remaining loci was evaluated applying Fdist2 [Beaumont and Nichols, 1996]. Fst estimates for each of the 36 loci were plotted against observed heterozygosity. The expected global Fst is calculated from the data as the average among loci weighted by their heterozygosities. The distribution of Fst as a function of heterozygosity was characterized by estimating the 0.025, 0.5, and 0.975 quantiles of the distribution by simulating 20,000 realization steps. To check for outliers locus-specific Fst estimates were plotted against these quantiles as a function of heterozygosity.
To check the data for any hidden population structure Bayesian model-based clustering using multilocus genotype data as implemented in STRUCTURE was applied [Pritchard et al., 2000]. The posterior probability was estimated for different number of putative clusters of the sample. An admixture linkage model was applied without using any prior information on population structure. In addition to taking into account that individuals may have mixed ancestry, and that different parts of their genome may actually belong to different clusters of the data set, the admixture linkage model also considers the possibility of correlations between markers due to linkage (“admixture linkage disequilibrium”). This method does not attempt to model the LD that occurs between very close markers within populations, and only the nine markers with very low pairwise LD were applied. A burning length of 100,000 simulations, followed by 500,000 simulations was used to get accurate parameter estimates. Ten iterations were performed for each assumed number of clusters (k from 1 to 8).
Association analysis stratified for collection site was carried out using the Cochran–Mantel–Haenszel test.
Meta-analysis was performed using the weighted z-score method [de Bakker et al., 2008]. Chi square values for each study were converted to a z-statistic which was signed to reflect the direction of the association given the reference allele. The z-statistics were weighted by the relative per-study sample size (ωi) and summed across studies. The weight ωi was calculated as √(Ni/Ntotal) where Ni was the sample size of each individual study, and Ntotal was the total sample size of all studies. The summary z-score (Zmeta) was converted to a 2-sided P-value.
RESULTS
Association Analysis of SZ and BPD in the UK/DK Sample
A total of 11 SNPs were successfully genotyped in the UK/DK sample (Table II), 7 of these were located within the BRD1 gene and 4 in the nearby gene ZBED4 (a brain-expressed gene of unknown function). Four of these SNPs were previously typed in the Scottish case–control sample [Severinsen et al., 2006] (marked with d in Table II). All SNPs had a call rate higher than 0.98. The genotype distribution did not deviate significantly from Hardy–Weinberg equilibrium in any of the groups (BPD, SZ, and controls). A sample quality filtering threshold of 0.90 was applied so that samples having more than 1 missing genotype out of the 11 were excluded from the analysis. Thirty-one samples were excluded on this basis.
| Gene | SNP (Sequenom) | Location (Mb)a | Type of SNP | Allelesb | MAF UK/DK | P-valuec | OR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 601) | Bipolar (n = 490) | SZ (n = 527) | Bipolar | SZ | Bipolar | SZ | |||||
| BRD1 | rs138820 | 48550082 | 3' UTR | T/C | 0.19 | 0.21 | 0.25 | 0.30 | 0.0014 | 1.12 | 1.39 |
| BRD1 | rs4468d | 48553656 | 3' UTR | T/C | 0.43 | 0.44 | 0.47 | 0.61 | 0.046 | 1.05 | 1.19 |
| BRD1 | rs138841 | 48567964 | Intron | C/A | 0.20 | 0.21 | 0.25 | 0.46 | 0.0042 | 1.08 | 1.34 |
| BRD1 | rs138855d | 48584486 | Intron | G/C | 0.18 | 0.18 | 0.21 | 0.93 | 0.062 | 1.01 | 1.22 |
| BRD1 | rs138864 | 48590716 | Intron | G/A | 0.18 | 0.19 | 0.21 | 0.64 | 0.043 | 1.06 | 1.24 |
| BRD1 | rs2239848d | 48602758 | Syn. | G/A | 0.01 | 0.01 | 0.02 | 0.80 | 0.37 | 0.90 | 1.40 |
| BRD1 | rs138880d | 48604615 | Promoter/intron | A/C | 0.18 | 0.19 | 0.21 | 0.45 | 0.043 | 1.09 | 1.24 |
| ZBED4 | rs910799 | 48664572 | Nonsyn. | G/A | 0.18 | 0.18 | 0.20 | 0.80 | 0.35 | 0.97 | 1.13 |
| ZBED4 | rs5770755 | 48666140 | Syn. | C/T | 0.18 | 0.19 | 0.21 | 0.72 | 0.072 | 1.04 | 1.21 |
| ZBED4 | rs6009917 | 48667348 | 3′ UTR | G/A | 0.003 | 0.007 | 0.010 | 0.10 | 0.025 | 2.93 | 3.92 |
| ZBED4 | rs4624 | 48667921 | 3′ UTR | G/A | 0.18 | 0.18 | 0.21 | 0.87 | 0.073 | 0.98 | 1.21 |
- SNP, single nucleotide polymorphism; Mb, megabases; MAF, minor allele frequency; OR, odds ratio; UTR, untranlated region; Nonsyn., non-synonymous; Syn., synonymous; SZ, schizophrenia.
- a According to the UCSC Genome Browser, March 2006 assembly (http://www.genome.ucsc.edu). P-values <0.05 in bold.
- b Major allele/minor allele on the +strand (http://www.genome.ucsc.edu).
- c Cochran–Armitage trend test.
- d SNPs which has previously been genotyped in a Scottish case–control sample [Severinsen et al., 2006].
Nominal significant association with SZ was observed for rs138820, rs4468, rs138841, rs138864, rs138880, and rs6009917 with a minimal P-value of 0.0014 (OR = 1.39) (Table II). Two of these SNPs (rs138820 and rs138841) reached the corrected experiment wide significance threshold of 0.010 for 5 independent tests calculated using the Nyholt method. Haplotype analysis revealed “risk” as well as “protective” haplotypes in SZ (Table III). “Risk” haplotypes over-represented among patients with SZ involved especially the BRD1 SNPs rs138820, rs4468, and rs138841 (minimal P-value of 0.0027), whereas “protective” haplotypes included the BRD1 SNPs rs138820, rs4468, rs138841, rs138855, rs138864, and rs2239848 (minimal P-value of 0.0081).
| Haplotype | Haplotype frequency | |||||||
|---|---|---|---|---|---|---|---|---|
| rs138820 | rs4468 | rs138841 | rs138855 | rs138864 | rs2239848 | Controls | SZ | Empirical P-valuesa |
| “Risk” haplotypes | ||||||||
| C | C | 0.19 | 0.25 | 0.0027 | ||||
| C | C | A | 0.19 | 0.24 | 0.0074 | |||
| C | A | 0.20 | 0.25 | 0.0073 | ||||
| “Protective” haplotypes | ||||||||
| T | T | 0.57 | 0.53 | 0.041 | ||||
| T | T | C | 0.57 | 0.53 | 0.044 | |||
| T | C | 0.57 | 0.53 | 0.043 | ||||
| T | C | G | 0.57 | 0.52 | 0.042 | |||
| C | G | 0.80 | 0.76 | 0.017 | ||||
| C | G | G | 0.80 | 0.76 | 0.0081 | |||
| G | G | 0.82 | 0.78 | 0.048 | ||||
- P-values <0.05 in bold.
- a Empirical haplotype-specific P-values calculated using PLINK.
None of the 11 Sequenom SNPs showed association with BPD.
Association Analysis of BPD in the UCL Sample
A total of seven SNPs on the Affymetrix 500K chip were located within the region of interest. In the UCL sample significant allelic association with BPD was observed for all seven SNPs with a minimal P-value of 0.0014 (Table IV). The effective number of independent tests was calculated to 3 and all seven SNPs reached the corrected experiment wide threshold of 0.017. There was no overlap in the 11 Sequenom SNPs typed in the UK/DK sample and the SNPs on the Affymetrix 500K chip analyzed in the UCL sample.
| Gene | SNP (Affy 500K) | Location (Mb)a | Type of SNP | Allelesb | MAF UCL | P-value | MAF WTCCCc | P-value | Meta-analysisd | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 510) | Bipolar (n = 506) | Controls (n = 14,311) | Bipolar (n = 1,864) | P-value | |||||||
| BRD1 | rs139811 | 48520208 | 3' UTR | G/T | 0.18 | 0.23 | 0.0099 | 0.20 | 0.21 | 0.114 | 0.027 |
| BRD1 | rs138830 | 48561093 | Intron | G/A | 0.08 | 0.12 | 0.0035 | 0.10 | 0.12 | 0.036 | 0.006 |
| BRD1 | rs138851 | 48579640 | Intron | T/C | 0.09 | 0.15 | 0.0039 | 0.11 | 0.12 | 0.051 | 0.009 |
| BRD1 | rs138863 | 48590277 | Intron | A/G | 0.08 | 0.12 | 0.0021 | 0.10 | 0.12 | 0.048 | 0.008 |
| BRD1 | rs138887 | 48611437 | Promoter | T/C | 0.09 | 0.13 | 0.0014 | 0.11 | 0.12 | 0.060 | 0.012 |
| BRD1/ZBED4 | rs5769750 | 48623599 | Promoter | G/A | 0.08 | 0.12 | 0.0022 | 0.10 | 0.11 | 0.040 | 0.006 |
| BRD1/ZBED4 | rs3788731 | 48630781 | Promoter | G/C | 0.08 | 0.11 | 0.0065 | 0.10 | 0.11 | 0.030 | 0.007 |
- SNP, single nucleotide polymorphism; Affy 500K; Affymetrix 5.0 SNP chip, Mb, megabases; MAF, minor allele frequency; UTR, untranslated region.
- a According to the UCSC Genome Browser, March 2006 assembly. P-values <0.05 in bold.
- b Major allele/minor allele on the +strand (http://www.genome.ucsc.edu).
- c Downloaded from the WTCCC website (http://www.wtccc.org.uk).
- d Weighted z-score method.
Support for association was also found in the WTCCC study of BPD, comparing patients with BPD to the large group of common controls (Table IV). In this dataset significant allelic association with BPD was observed for four of seven SNPs with a minimal P-value of 0.030.
Linkage Disequilibrium
In order to evaluate whether the two SNP sets applied in this study captures the same genetic variation, the haplotype block structure on 22q13.33 and the degree of LD between the Sequenom and the Affymetrix SNPs were assessed in the CEU population using data from the HapMap project (phase II) (http://www.hapmap.org). The analysis showed a high degree of LD (measured as D′) within and between BRD1 and the neighboring ZBED4 gene in the CEU population, with the genes located in two closely connected haplotype blocks (data not shown). Assessment of the r2 values for specific SNP pairs showed a high or moderate degree of LD between the Sequenom SNPs typed in the UK/DK sample, a high degree of LD between the Affymetrix SNPs genotyped in the UCL sample, whilst the LD between the SNPs in these two separate SNP-sets were relatively low (r2 < 0.5) (Fig. 1). This collectively indicates that the two SNP sets capture the underlying haplotypes with different statistical power.
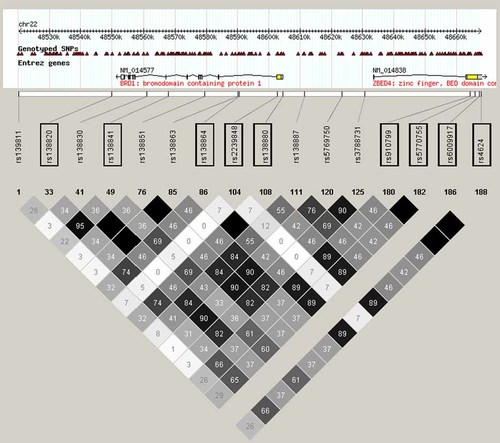
The degree of linkage disequilibrium (r2) between Sequenom SNPs (boxed) and Affymetrix, 500K SNPs (not boxed) in the CEU population. rs6009917 was monomorphic in the CEU population, (no r2 values). Two Sequenom SNPs were not genotyped in the HapMap project (not included). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In the UK/DK sample (n = 1,621), high or moderate degree of LD was found between the majority of the genotyped SNPs. The exception was rs4468, which displayed r2 values lower than 0.33 for all pairs tested, and rs2239848 and rs6009917, which had very low minor allele frequencies. The SNP with the strongest association in the UK/DK SZ sample (rs138820) displayed high LD with rs138841 (r2 = 0.95) and moderate LD with rs138864, rs138855, and rs138880 (r2 = 0.83, 0.82, and 0.81, respectively).
Imputing
Imputation of genotypes in the UK/DK sample (n = 1,621) was performed using data from six Sequenom SNPs that had also been genotyped in the HapMap phase I in the CEU population. In the 118 kb region of interest, 84 SNPs were imputed with 25 of these being predicted with a likelihood of 90% or higher (quality score above 0.90). Of the 25 imputed SNPs, 23 displayed tight or relatively tight LD to one of the genotyped SNPs (r2 > 0.75), while 2 SNPs showed less LD (r2 ≈ 0.40). The genotype distribution for all 25 imputed SNPs did not deviate significantly from Hardy–Weinberg equilibrium. Allelic association with SZ was found for imputed SNPs rs138823 (P = 0.0092) and rs138843 (P = 0.0092) (Fig. 2). None of the imputed SNPs showed allelic association with BPD.
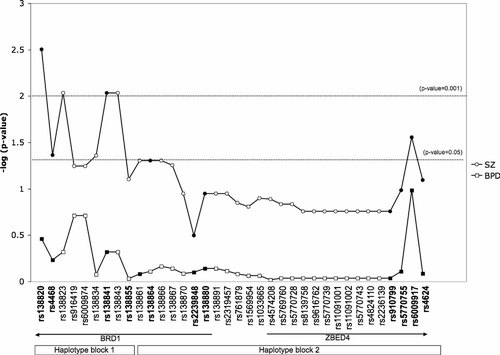
Significance of association with BPD and SZ in the UK/DK sample for Sequenom SNPs (filled symbols) and imputed SNPs (open symbols). Haplotype blocks were estimated using Haploview and genotypes from the CEU population.
Population Stratification Analysis
Analysis of the SNPs selected for analysis of population substructure using the exact test for genetic homogeneity showed that there was no global genetic heterogeneity between samples (P = 0.45), and the pairwise differentiation test showed that there was genetic homogeneity between all pairs of samples comparing the UK and DK samples.
Power analysis showed that nine SNPs, with the observed allele frequencies and sample size of the current study and using a significance threshold of 0.05, is enough to detect a genetic differentiation between samples corresponding to an Fst of 0.0015 with a 90% probability (data not shown). Despite the rather low number of loci we have sufficient power to detect a reasonably small amount of genetic differentiation applying an exact test for genetic homogeneity. None of the 36 genotyped SNPs appeared as outliners when locus-specific Fst estimates were evaluated against a Fst null distribution as a function of marker heterozygosity generated by 20,000 realization steps and an expected Fst of 0.001 as implemented in Fdist2 (data not shown). Applying STRUCTURE on the nine SNPs revealed k = 1 as the most likely number of clusters of this sample (data not shown), thereby suggesting that there is no hidden genetic substructure in the sample. The power of this approach is, however, limited when applying only nine markers.
In addition, the UK/DK sample was analyzed applying stratification for site of collection. This analysis showed similar results as reported in Table II, indicating that the heterogeneity arising from site was negligible.
Meta-Analysis
In a meta-analysis of BPD and SZ across the Scottish sample analyzed in the initial study [Severinsen et al., 2006] and the present UK/DK sample for the four SNPs genotyped in both studies (rs4468, rs138855, rs2239848, and rs138880) we found significant association with SZ for rs4468 (combined P = 0.001) and rs138880 (combined P = 0.002). No significant association with BPD was found with the four markers (best marker rs138880, combined P = 0.09).
In a meta-analysis of BPD across the UCL sample and the WTCCC sample for the seven Affymetrix SNPs, significant association to BPD was found with all SNPs yielding combined P-values ranging from 0.006 to 0.027 (Table IV).
We were unable to include all samples in a single meta-analysis, as the same SNPs were not available for all the samples.
DISCUSSION
We here report replication of association for variations in the BRD1 gene with SZ and BPD.
We found nominal significant single marker association with SZ for six BRD1/ZBED4 SNPs in a Caucasian case–control sample (the UK/DK sample). For the two SNPs rs4468 and rs138880, which were previously found to be associated with SZ in the Scottish case–control sample investigated by our group, we found allele wise replication with the minor allele over-represented among patients with SZ in both populations. The two SNPs that survived the correction for multiple testing (rs138820 and rs138841) are tagging the same underlying risk variant due to the existence of almost complete LD between them (almost perfect proxi). The relatively low LD between rs4468 and all other SNPs suggests that two or more underlying risk haplotypes exists. In addition we found association with SZ for risk as well as protective haplotypes. We did not replicate the association with SZ and BPD with the specific two-marker risk haplotype identified in the Scottish case–control sample, which suggest the presence of different underlying susceptibility variants in the Scottish and the UK/DK populations.
In the UK/DK sample we found no association with BPD. However, analysis of data on seven BRD1 Affymetrix SNPs obtained from a parallel BPD GWA study of the UCL bipolar sample, which includes all the English subjects of the UK/DK samples, identified significant single marker association with BPD for all SNPs in BRD1. None of these seven SNPs were typed in the Danish subjects of the UK/DK sample. As a consequence of the relative low degree of LD (r2 < 0.5) between the two different SNP sets (the 11 UK/DK Sequenom SNPs, and the 7 UCL Affymetrix SNPs) we were unable to impute any of the Affymetrix SNPs from the Sequenom SNPs and visa versa. Because of the LD structure, the two platforms assess the underlying genetic variations with different statistical power. Specifically the Affymetrix SNPs seem to be more efficient in tagging the potential BPD risk haplotypes, while the UK/DK sample appeared to be underpowered to identify the risk haplotypes for BPD when using the 11 Sequenom SNPs.
In the present study there was an overlap in patient with BPD and controls between the UK/DK and the UCL sample. We found no association with BPD in the UK/DK sample; accordingly we only report BPD association from the UCL sample. Therefore, all significant association results reported in this study were obtained from independent, non-overlapping samples.
Support for association of the Affymetrix BRD1 SNPs with BPD was found in the WTCCC GWA data set showing allelic replication of four of the seven SNPs and a trend towards association for the remaining three SNPs. However, in an American case–control sample (STEP-BD sample) which was also analyzed in the GWA study of BPD by Sklar and co-workers no significant association was seen in the BRD1 gene. The lack of replication in the STEP-BD sample might be due to the smaller sample size (and lower power) as compared to the WTCCC study, or sample heterogeneity.
Our data suggest that BRD1 might be a shared risk gene for BPD and SZ. In the initial study using a Scottish case–control sample, a specific two-marker haplotype located in the 5′ region of the gene was over-represented in both patients with BPD and SZ, suggesting that a common risk allele for the two phenotypes existed [Severinsen et al., 2006]. We were not able to replicate the association to BPD or SZ with this specific haplotype. In the present study, the most significant association with SZ was observed for SNPs in the 3′ untranslated region of the BRD1 gene, while association with BPD was seen in a different set of SNPs across the entire gene. As the haplotypes implicated in BPD and SZ in this study are somewhat related (judged by the LD structure) it may indicate the existence of a shared risk variant in BRD1. However, a study using systematically selected tag SNPs across the entire region in a large independent sample is warranted to confirm this.
Due to high LD in the region harboring BRD1, we included SNPs located within the ZBED4 gene in this replication analysis. Analysis of genotyped SNPs as well as imputed genotypes showed the strongest association with markers in BRD1. However, due to the high LD we cannot completely rule out ZBED4 as a positional candidate gene for SZ and BPD. Imputing was performed in the UK/DK sample to obtain genotypes of untyped markers, thereby potentially increasing information and power. We had a limited number of independent SNPs, and imputing did not point at any SNPs with improved significance over the already genotyped SNPs.
In this study we merged two Caucasian case–control samples with very similar ascertainment and diagnostic criteria. The benefit was an increased sample size and thereby a higher power in the study, while the drawback was an increased risk of population stratification and increased genetic heterogeneity, the latter lowering the chance of identifying potential rare risk variants. Concerning the potential population stratification we did not find any evidence of genetic differentiation between the UK and the DK sample. Also no evidence of heterogeneity arising from site was found.
Numerous studies have found evidence of linkage to chromosome 22q in BPD and SZ samples, and several of these found evidence of linkage at the very telomeric region containing BRD1 (22q13.3), in particular in SZ samples [Stober et al., 2000; Mowry et al., 2004; Takahashi et al., 2005]. Association with SZ and BPD has been reported for the MLC1 gene [Verma et al., 2005], which is a gene located <0.5 Mb telomeric to BRD1. Furthermore, a recent study reported that the marker D22S526 (located around 0.3 Mb centromeric to BRD1) showed significant distortion in allelic transmission in 27 SZ families [Condra et al., 2007]. We recently reported association with SZ and BPD for variations within the BRD1 gene in a Scottish sample [Severinsen et al., 2006]. The present study obtained allelic replication in SZ in a large UK/DK sample. For BPD a different set of BRD1 SNPs showed association, and most of these SNPs showed the same allelic association in the WTCCC GWA data set comprising more than 15,000 individuals. Thus, these results provide strong evidence supporting that the BRD1 locus is involved in susceptibility to SZ in particular and also—but less unambiguously—to BPD.
Several large GWAS for BPD and SZ has recently been published including [Ferreira et al., 2008; Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009]. None of these studies report the BRD1 gene as one of their top hits. However, in general there are high degree of diversity among the top hits across the different GWAS and it would be interesting to combine the BRD1 results in a large meta-analysis when these data become available.
BRD1 is a brain-expressed gene, which has a bromodomain and a FAM domain and by sequence similarity it classified as a potential regulator of transcription. The gene is not well investigated and the function is not yet understood. Interestingly, the BRD1 gene was recently found by selective sweep analysis to have been subjected to positive selection in recent human evolution [Crespi et al., 2007]. Furthermore, BRD1 is located in the close vicinity of a “human accelerated region” (HAR33), recently identified by Pollard et al. 2006. Human accelerated regions (HARs), first described in 2006, are a set of 49 segments of the human genome, which are conserved throughout vertebrate evolution but are strikingly different in humans. It is hypothesized that these regions may contain genes or regulatory units that have contributed to the development of human neuroanatomy, language, and complex thought [Pollard et al., 2006]. We have previously reported that the BRD1 transcript is highly regulated during fetal brain development [Severinsen et al., 2006]. These findings collectively suggest that BRD1 could have a key function in the development of brain regions important for human evolution. It will be interesting to further investigate the molecular BRD1 pathways in normal brain development as well as BPD and SZ pathophysiology.
Acknowledgements
We thank the patients and control individuals who participated in the study. This study was supported by grants from the Danish Medical Research Council, the Danish Strategic Research Council, the Novo Nordisk Foundation, the Villum Kann Rasmussen Foundation, the Faculty of Health Sciences at Aarhus University, the Desirée and Niels Ydes Foundation, and the Psychiatric Research Foundation.




