PER2 variantion is associated with depression vulnerability†
How to Cite this Article: Lavebratt C, Sjöholm L, Partonen T, Schalling M, Forsell Y. 2009. PER2 Variantion Is Associated With Depression Vulnerability. Am J Med Genet Part B 153B:570–581.
Abstract
The circadian clock is driven by transcription–translation feedback loops and regulates rhythms that approximate the 24-hr day–night cycle or light–dark transitions. Disruptions of the circadian rhythms are common in depressed patients, expressed for example as sleep disturbances. Genetic variations in core circadian genes may in part explain these abnormalities. To investigate whether genetic variation in core circadian genes associates with vulnerability to depression, we genotyped 18 genes in a Swedish population based sample. Genetic variations indicative of association with depression, or with winter depression in our previous study, were tested for association to depression in a second Swedish depression-control sample set. PER2 genetic variation was associated with depression vulnerability, and this genetic risk did not seem to require exposure to potential sleep disturbance factors such as negative life event or financial strain that are known to increase the risk for depression. Polymorphisms in the circadian genes NPAS2, ARNTL, and RORA were also suggested to contribute to depression vulnerability. The findings we report for PER2, ARNTL, and RORA are supported by at least two of the three sample sets. In conclusion, genetic variation in PER2 is associated with depression vulnerability a Swedish population-based sample. More studies are needed to determine if this is the case also for NPAS2, ARNTL, and RORA. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Rhythms that approximate the 24-hr day–night cycle are called circadian. They are driven by cell-autonomous transcription–translation feedback loops in the brain and body [Takahashi et al., 2008], that are orchestrated by the principal clock that is located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus [Liu et al., 2007]. The major output targets of SCN neurons are the hypothalamic paraventricular and dorsomedial nuclei. The SCN output influences the timing of their hormone release and neuronal signaling regulating physiological rhythms. In mammals, the core circadian pathway is a negative feedback loop where the transcription factors CLOCK and NPAS2 during the day form a complex with ARNTL and ARNTL2 that binds DNA and activates the transcription of PER1/PER2 and CRY1/CRY2 genes. The encoded PER and CRY proteins heterodimerize, translocate to the nucleus and block their own transcription by interaction with CLOCK/NPAS2-ARNTL/ARNTL2 dimers. The PER-CRY complexes are gradually phosphorylated to target them for degradation. Key kinases for the phosphorylation are CSNK1D and CSNK1E. Also PER3 contributes to the clock output but seems not to have a critical role in this loop [Shearman et al., 2000]. In addition to this primary loop, a secondary negative feedback loop exists and is thought to control the stability of the core clock. In this secondary loop the ARNTL-CLOCK complex activates the nuclear hormone receptors REV-ERB (NR1D1 and NR1D2) and ROR (RORA, RORB, and RORC). These transcription factors compete for binding to the ARNTL promoter, REV-ERB being repressors and ROR activators. There are several other clock components with a less defined role. Clock genes are expressed in many tissues throughout the body, and almost every tissue in the body has the capacity to generate circadian oscillations that are subsequently entrained by the master clock. Recent studies indicate that some of these genes have effects beyond the circadian system [for review, see Takahashi et al., 2008].
Disruptions of the circadian rhythms are common in depressed patients in several psychological and physiological domains [for review, see Germain and Kupfer, 2008]. For example, of the depressed patients, approximately 90% have difficulty falling asleep, staying asleep or have early morning awakening. The rapid-eye-movement sleep and the slow-wave sleep, the former speeding up and the latter slowing down the firing rate of the cells in the SCN, are also disturbed in depression. Both delays and advances in the phase positions of the circadian rhythms as well as reduced circadian rhythm amplitudes [caused by the desynchronization of the rhythms, Ukai et al., 2007] have been reported associated with non-seasonal and seasonal depressive episodes [for review Souêtre et al., 1989; Bunney and Potkin, 2008; Germain and Kupfer, 2008]. Of the behavioral phenotypes of the intrinsic circadian clockwork, the preference to schedule the daily activities to the evening hours (eveningness) has been demonstrated to have a delayed phase position and to associate with depressive episodes features with a higher severity [Chelminski et al., 1999; Gaspar-Barba et al., 2009].
Therefore, genetic variations in the core circadian genes may in part explain circadian rhythm abnormalities in mood disorder, and associate with mood disorder [for review, see McClung 2007]. A limited number of reports exist from studies of genetic variations in circadian clock genes in mood disorders. NPAS2, PER2, and ARNTL gene variations were associated with seasonal affective disorder [Johansson et al., 2003; Partonen et al., 2007]. A CLOCK gene variation was associated with bipolar disorder [Shi et al., 2008], and on single SNP basis bipolar disorder was associated with genetic variations in the NR1D1, ARNTL, and PER3 genes [Nievergelt et al., 2006; Kripke et al., 2009]. In bipolar disorder, morningness–eveningness that corresponds with the circadian period and phase position [Duffy et al., 1999], was associated with polymorphisms in the PER3 and CSNK1E genes [Kripke et al., 2009]. A recent meta-analysis of the data from three genome-wide association studies (GWAS) of bipolar disorder was integrated with human and animal model expression data and identified the circadian genes ARNTL, RORA, and RORB, whose significant associations were replicated in another GWAS [Le-Niculescu et al., 2008].
To investigate whether genetic variations in circadian genes associate with vulnerability to unipolar depression, we genotyped 18 circadian genes in a Swedish population based sample. The strategy was to first identify genetic variations indicative of association in a depression sample as compared to controls with mental resilience to adverse life-events. Those variations, and variation previously indicated to be associated with winter depression in a Swedish cohort [Sjöholm et al., manuscript], were then tested in a second Swedish depression-control sample set. Environment is known to contribute to depression vulnerability. To determine whether identified genetic risk variants constitute depression risk factors independent of potential sleep disturbing environmental stressors, the genetic risks were subsequently studied in those individuals reporting no negative life event or financial strain during last year. Both these stressors have previously been reported to be risk factors for depression [Lehtinen and Joukamaa, 1994].
MATERIALS AND METHODS
Subjects
The subjects derive from the PART study which is a longitudinal population based study of mental health, work and relationships ongoing in Stockholm, Sweden [www.folkhalsoguiden.se, Hällström et al., 2003]. From the Population Registry, 19,742 Swedish nationals, 20–64 years and residing in Stockholm County 1998–2000 were randomly selected. Totally 10,441 persons (53%, 4,643 men and 5,798 women), filled in an extensive questionnaire. The first part of the questionnaire included questions on childhood conditions, demographic characteristics, financial status, social network, negative life events, somatic health, and use of drugs. The second part included screening instruments for psychiatric disorders; Sheehan Patient-Rated (Panic) Anxiety Scale [Sheehan, 1983], the Yale-Brown Obsessive-Compulsive Scale [Goodman et al., 1989], symptoms of social phobia and agoraphobia according to Marks and Mathews 1979, the Major Depression Inventory [Bech and Wermuth, 2000], eating disorders according to Beglin and Fairburn 1992, the World Health Organization Short Disability Assessment Schedule (WHO DAS-S) [Janca et al., 1996], and harmful alcohol use according to Alcohol Use Disorder Identification Test (AUDIT) [Saunders et al., 1993]. In addition, a well-being scale was included [Bech et al., 1996] [for detailed information of the study see Hällström et al., 2003].
In a second wave 3 years later 8,613 of the 10,441 participants filled in a second almost identical questionnaire as well as the Swedish Universities Scales of Personality (SSP) [Gustavsson et al., 2000]. Detailed attrition analyses for both waves, done by linking the personal identification number (given to all Swedish citizens) of each participant to several official registries, assured that relationships between living conditions and psychiatric disorders was likely to be identified accurately. Odds ratios for psychiatric diagnoses in the inpatient registries were quite similar among participants and non-participants when related to gender, age, income, education, country of birth, number of days with sickness-allowance and socio-economic group [Lundberg et al., 2005; Bergman et al., in press].
Definition of Depression, Mental Resilience, and Control
Of the 8,613 individuals participating in both waves, individuals with a depression diagnosis, or characteristics fulfilling the criteria for being a control subject were selected. Depression was defined according to DSM-IV using the Major Depression Inventory according to the authors' instructions [Bech and Wermuth, 2000]. A depression diagnosis included major depression, dysthymia and mixed anxiety depression. Individuals with a depression in at least one wave qualified for the depression group. Controls were persons with no symptom of psychopathology in any of the two waves defined as having no symptom of anxiety, social phobia, agoraphobia, obsessive-compulsive disorder, eating disorder, use of illicit drug, depression or social disability due to psychological problem. Moreover, the controls stated they had never received health care for psychiatric disorder or nervous discomfort. An additional control group of mentally resilient persons were those persons who fulfilled the criteria for controls, and in addition had a wellbeing score ≥ the score average in controls and mental resilient group combined (score ≥21, out of a maximum of 30) in both waves, despite the occurrence of one or more potentially traumatic negative life event within 12 months before the second wave [Forsell and Lavebratt, manuscript].
Analysis was initially performed on Sample set I: Depression cases versus mentally resilient persons. Genetic findings from Sample set I using mentally resilient controls could reflect variants associated with wellbeing rather than disease. Therefore, the findings from Sample set I were followed up in Sample set II: Depression versus controls, as well as compared with our previous findings in winter depression [Sjöholm et al., manuscript]. The depression cases were the same in the Sample set I and II, but the mental resilience and controls groups have no overlap regarding individual DNAs.
Environmental Factors
Serious stressful life events, potentially negative, affecting primarily the informant were recorded: A list of 11 potential negative life events was presented and the persons stated if they had occurred or not during the last 12 months. The life events were all serious for example serious illness/injury, serious abuse/crime victim, unemployment, homelessness, severe conflicts with spouse or others.
Financial strain was defined as not having enough money for food and housing. The individuals scoring positive are partially distinct from those with serious stressful financial life event; that is, there are those with financial strain not preceded by any financial life event during the last 12 months, and there are those with financial life event not resulting in financial strain.
SNP Selection and Genotyping
Saliva samples for DNA were collected through saliva kit (Oragene*DNA, DNA Genotek, Inc., Ottawa, Ontario, Canada) as described in Sjöholm et al. 2009b and DNA was extracted using the Oragene Purifier. This study is in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The ethical committee of Karolinska Institutet approved the study and specified written consent was obtained from all the participants. Genotyping was performed on 459 depression cases, 462 mentally resilient subjects and 926 controls, which comprise a random selection of the 484 cases, 481 mental resilience subjects, and 1,877 controls.
A total of 115 SNPs from 18 known circadian clock genes [Ukai-Tadenuma et al., 2008] were genotyped (Table I). The SNPs were selected using HapMap database [The International HapMap Consortium, 2005] to cover haplotype-tagging single nucleotide polymorphisms (SNPs) within each gene. We used the cutoff value for r2 at 0.8 and for minor allele frequency (MAF) at 0.1. We used the tagger tool of Haploview program to further diminish the number of needed SNPs for genotyping [de Bakker et al., 2005]. This tool combines the pairwise r2 method with multimarker haplotype approaches and it searches multimarker predictors as surrogates for single tag SNPs and helps to select SNPs that cover most efficiently the common variants. We also selected polymorphic non-synonymous SNPs to cover the potentially functional variants. For the large RORA and NPAS2 genes we selected the tagging SNPs throughout genes and tried to favor the ones that cover large areas. Haplotype tagging SNP coverage was 66% and without the two large genes (RORA and NPAS2) the coverage was 72%.
| Gene | Gene name | Location | SNPs typed |
|---|---|---|---|
| PER1 | Period homolog 1 (Drosophila) | 17p13.1 | rs2289591, rs2253820, rs3027188, rs885747, rs2518023 |
| PER2 | Period homolog 2 (Drosophila) | 2q37.3 | rs881933, rs934945, rs6431590, rs3739064, rs11894535, rs10462023, rs2304672, rs4663302 |
| PER3 | Period homolog 3 (Drosophila) | 1p36.23 | rs3753503, rs228729, rs228682, rs228642, rs1891217, rs17374292, rs12035969, rs10462021 |
| RORA | RAR-related orphan receptor A | 15q22.2 | rs2290430, rs12914584, rs2028122, rs4775281, rs4774370, rs1863270, rs11637301, rs341373, rs8027829, rs16943429, rs1568717, rs893287, rs4774388, rs1816624, rs6494251, rs10438343 |
| CRY1 | Cryptochrome 1 (photolyase-like) | 12q23.3 | rs2287162, rs2287161, rs10861683, rs11113179, rs3809237 |
| CRY2 | Cryptochrome 2 (photolyase-like) | 11p11.2 | rs10838524, rs7123390, rs10838527, rs3824872 |
| ARNTL | Aryl hydrocarbon receptor nuclear translocator-like | 11p15.2 | rs2279287, rs7950226, rs10766074, rs1982350, rs6486121, rs1562438, rs2290036, rs1868049, rs11022778, rs3816358, rs4757151, rs2278749, rs3897902, rs969485 |
| ARNTL2 | Aryl hydrocarbon receptor nuclear translocator-like 2 | 12p11.23 | rs10842905, rs7137588, rs4964052, rs922270, rs17413842, rs4964060, rs7304939, rs12299407, rs1037921, rs4931075, rs2289709 |
| NPAS2 | Neuronal PAS domain protein 2 | 2q11.2 | rs6722909, rs1811399, rs11541353 (S471L), rs12712083, rs2117714, rs6725296, rs3820785, rs12712085, rs17025078, rs2305160, rs1374324 |
| CLOCK | Clock homolog (mouse) | 4q12 | rs10462028, rs1801260, rs11932595, rs6850524, rs4864548 |
| NFIL3 | Nuclear factor, interleukin 3 regulated | 9q22.31 | rs1619450, rs10991925, rs2440589, rs968821, rs813498 |
| TIMELESS | Timeless homolog (Drosophila) | 12q13.2 | rs2291739, rs2291738, rs7486220, rs1082214 |
| TIPIN | TIMELESS interacting protein | 15q22.31 | rs3759785, rs3759786, rs2063690, rs8031897 |
| CSNK1E | Casein kinase 1, epsilon | 22q13.1 | rs135745, rs2075984, S408N, rs5750581, rs7289981 |
| NR1D1 | Nuclear receptor subfamily 1, group D, member 1 | 17q21.1 | rs2314339, rs2071427, rs2269457, rs2071570 |
| DBP | D site of albumin promoter (albumin D-box) binding protein | 19q13.33 | rs3745733 |
| BHLHB2 | Basic helix-loop-helix domain containing, class B, 2 | 3p26.2 | rs908078, rs11130215, rs2137947 |
| BHLHB3 | Basic helix-loop-helix domain containing, class B, 3 | 12p12.1 | rs1048155, rs3809140 |
- Symbol approved by the HUGO Gene Nomenclature Committee (HGNC) database (http://www.genenames.org/), location according to the Ensembl cytogenetic band (http://www.ensembl.org/), rs# from the NCBI dbSNP BUILD 129 database (http://www.ncbi.nlm.nih.gov/).
The genotyping was performed using Sequenom's iPLEX™ biochemistry in multiplexes of 24–34 with the MassARRAY System (Sequenom®, San Diego, CA). As quality controls, overall 2.5% of the samples were genotyped in duplicates. The genotypes of these replicas were all congruent. The Hardy–Weinberg equilibrium was also monitored.
SNP and DNA Exclusion
Out of the 115 SNPs genotyped the CSNK1E SNP S408N (Table I) was non-polymorphic, and 12 SNPs had a success rate <90% for each of the two sample sets excluding them from association analysis (rs4774370, rs1863270, rs8027829, rs7123390, rs4931075, rs3027188, rs2253820, rs2287161, rs4864548, rs11932595, rs2278749, rs2291738). Exclusion from analysis due to Hardy–Weinberg disequilibrium in the control sample (P < 0.05) was the case for five SNPs in Sample set I (rs3745733, rs2063690, rs228729, rs228642, and rs2287162), and three SNPs in Sample set II (rs341373, rs3759785, and rs1562438). Furthermore, individual DNA samples that were successful for <80% of the SNP assays were excluded from the association analyses being 27 depression cases (5.9%), 28 mental resilience samples (6.1%), and 46 controls (5.0%). The gender and age characteristics of the individuals with successfully genotyped DNAs are given in Table II.
| Age: mean ± SD, n (range) | % females | ||
|---|---|---|---|
| Females | Males | ||
| Depression | 45.0 ± 12.1, 311 (21–67) | 43.7 ± 11.5, 117 (23–66) | 72.7 |
| Mental resilience | 42.6 ± 11.7, 277 (20–66)a | 45.2 ± 11.7, 154 (23–66) | 64.3a |
| Controls | 46.8 ± 11.9, 513 (21–67) | 47.7 ± 12.4, 364 (22–67)a | 58.5a |
- Samples successful for <80% of SNP assays are excluded. Age as of year 2000 is given.
- a P < 0.05 as compared to depression.
Statistical Analysis
Differences in age and gender between groups were tested using the Kruskal–Wallis test and Fisher's exact test, respectively. Gender differed between the depression and the mentally resilient group (Sample set I, P = 0.02), whereas an age difference between groups existed only for one gender (Table II). First, all SNPs were analyzed for allele frequency difference between depression cases and mentally resilient samples (Sample set I) using logistic regression. To obtain empirical significance, a permutation test (10,000 permutations) was performed (Table III). Gender was incorporated as covariate in the logistic regression analysis for allele frequency association. Since age did not differ between genotypes of the SNPs with indicated allelic association to depression (P > 0.05) these findings were unlikely to be false positive due to intergroup age difference. There was a very high similarity between the gender corrected effect size (OR) and the effect size when no gender was corrected for (Table III). Hence, neither age nor gender was controlled for in the subsequent genotype and haplotype analyses.
| SNP | Function | Gene | Alleles | MAF (A/U) | OR [95% CI]a | P-valuea | OR [95% CI]b | Empirical, Pc |
|---|---|---|---|---|---|---|---|---|
| Sample set I: depression compared to mental resilience | ||||||||
| rs6431590 | Intron 17 | PER2 | C/T | 0.35/0.30 | 1.25 [1.01–1.52] | 0.036 | 1.24 [1.01–1.52] | 0.041 |
| rs3739064 | Intron 8 | PER2 | G/A | 0.27/0.22 | 1.30 [1.05–1.62] | 0.018 | 1.32 [1.06–1.64] | 0.014 |
| rs10462023 | Intron 3 | PER2 | T/C | 0.35/0.42 | 0.74 [0.61–0.91] | 0.0033 | 0.76 [0.62–0.92] | 0.0058 |
| rs2304672 | Exon 2 5′UTR | PER2 | G/C | 0.13/0.083 | 1.50 [1.11–2.03] | 0.0087 | 1.59 [1.16–2.18] | 0.0043 |
| rs1374324 | 3′ near gene | NPAS2 | A/G | 0.36/0.41 | 0.77 [0.64–0.94] | 0.011 | 0.76 [0.63–0.93] | 0.0063 |
| rs2028122 | Intron 2 | RORA | A/G | 0.34/0.39 | 0.81 [0.67–0.99] | 0.037 | 0.80 [0.66–0.98] | 0.031 |
| Sample set II: depression compared to controls | ||||||||
| rs2290036 | Intron 6 | ARNTL | C/T | 0.09/0.07 | 1.37 [1.01–1.85] | 0.043 | 1.36 [1.00–1.84] | 0.041 |
| rs2028122 | Intron 2 | RORA | A/G | 0.34/0.38 | 0.84 [0.71–0.99] | 0.044 | 0.83 [0.70–0.99] | 0.033 |
- Alleles, minor allele first. OR, proportion of minor versus major allele among affected (A)/proportion of minor versus major allele among non-affected (U).
- a Logistic regression with gender as covariate. P < 0.05 are shown.
- b No covariate.
- c Point-wise P-value from 10,000 permutations with no covariate (EMP1).
Power to detect allele frequency difference was ≥0.8 for OR ≥1.5 at an allele frequency of ≥0.3, OR ≥1.6 at an allele frequency of ≥0.2, and OR ≥1.75 at an allele frequency of ≥0.1. SNPs with suggestive allele frequency difference (P < 0.05) were analyzed for genotype association. Association calculations were performed with PLINK v.1.04 [Purcell et al., 2007]. The LD measure D′ between the SNPs within each gene was calculated using Haploview v.3.2. Haploview uses an approach similar to the partition-ligation-EM algorithm described by Qin et al. 2002. Haplotype blocks were constructed in the mentally resilient group, using LD block parameters defined in Gabriel et al.'s 2002 and the D′ confidence interval algorithm in Haploview [Barrett et al., 2005]. Test for haplotype frequency difference in Sample set I was performed for the haplotype blocks harboring the SNPs with P < 0.05 for allele association using PLINK.
A similar procedure has been performed by us for the identification of possible association between this set of SNPs and winter depression [Sjöholm et al., manuscript]. The SNPs showing an indication of allele frequency association to winter depression in a Swedish sample in that previous report (Table IV), or to depression versus mental resilience (Sample set I) from the analysis described above, were tested for association with depression compared to controls (Sample set II). Sample set II was analyzed using the strategy as outlined above, referring to LD maps and block structures derived from this control sample. Power to detect allele frequency difference was ≥0.8 for OR ≥1.4 at an allele frequency of ≥0.3, OR ≥1.5 at an allele frequency of ≥0.2, and OR ≥1.6 at an allele frequency ≥0.1.
| SNP | Allele frequency | P-value | ||||
|---|---|---|---|---|---|---|
| OR | P-value | Cochran-Armitage trend | Minor allele dominanta | Minor allele recessivea | Haplotype | |
| ARNTL | ||||||
| rs2290036 | 1.72 [1.04–2.84] | 0.034 | 0.010 | 0.006 | 1.0 | 0.0011b |
| rs3816358 | 1.73 [1.94–6.26] | 0.016 | 0.0012 | 0.002 | 0.55 | |
| PER2 | ||||||
| rs934945 | 0.46 [0.27–0.76] | 0.0026 | 0.0079 | 0.025 | 0.026 | 0.0052c |
| RORA | ||||||
| rs12914584 | 1.46 [1.03–2.07] | 0.032 | 0.093 | 0.034 | 0.79 | ND |
| rs2028122 | 1.42 [1.03–1.95] | 0.030 | 0.11 | 0.13 | 0.31 | |
| rs893287 | 1.46 [1.06–1.20] | 0.020 | 0.11 | 0.12 | 0.34 | |
| CSNK1E | ||||||
| rs2075984 | 0.70 [0.52–0.95] | 0.020 | 0.079 | 0.042 | 0.40 | ND |
- Allele 1 is the minor allele.
- ND, not determined since not part of a block.
- a Two-sided Fischer's exact test was used if any cell <5.
- b rs1868049–rs11022778–rs3816358 (CTA OR = 1.89).
- c rs934945–rs6431590–rs3739064 (TTA OR = 0.54).
Haplotype blocks indicated to be associated with winter depression, or depression in Sample set I, (i.e., those haplotypes in the haplotype blocks given in Tables IV and VI) were tested for association to depression in Sample set II, even if no single SNP in that block was indicated to be associated with depression in Sample set II. Generally, nominal P values below 0.05 are shown in the tables.
P values are presented uncorrected nominal (P) and corrected (Pcorr) for multiple comparisons. Threshold for significance and Pcorr was calculated using a Bonferroni correction considering the partial LD between several markers [Nyholt, 2004; Gao et al., 2008]. The significance level was based on the number of SNP-groups defined by D′ > 0.80. For the mental resilience group the number of SNP-groups was 50, hence for Sample set I P = 0.001 corresponded to Pcorr = 0.05 and P ≤ 0.001 (=0.05/50) was regarded significant. For the control group in Sample set II the number of SNP-groups defined by D′ > 0.80 was 9 from the 14 SNPs analyzed (CRY2: rs10838524, rs10838527, rs3824872, ARNTL: rs2290036, rs3816358, RORA: rs12914584, rs2028122, rs893287, CSNK1E: rs2075984, NPAS2: rs1374324, PER2: rs6431590, rs3739064, rs10462023, rs2304672). Difference in allele frequency of SNPs between depression and controls (in Sample set II), and hence support of finding in Sample set I was regarded significant if P ≤ 0.0055 (=0.05/9). Haplotype analysis in Sample set II implicated genotyping for additional SNPs in two blocks (ARNTL: rs1868049, rs11022778, and NPAS2: rs2305160, rs1374324). That is, for the haplotype analysis in Sample set II P ≤ 0.004 (=0.05/(9 + 2)) was regarded as significant. The SNP rs1374324 was included in NPAS2 block 2 haplotype even though not included in any block, since it was suggestively associated to depression as a single SNP, and located nearby the block 2. The SNP rs934945 was included in PER2 block 1 haplotype in Sample set II since it belonged to that block in the mentally resilient group. Likewise, the PER2 SNP rs10462023 was included in block 2 haplotype in Sample set II since there was a very high D′ to rs2304672 (D′ > 0.96) and it belonged to that block in the mentally resilient group.
RESULTS
To identify genetic variation that may associate with variation in depression (major depression, dysthymia, and mixed anxiety depression) in the Swedish population, 115 SNPs from 18 genes known to be involved in circadian rhythm regulation (Table I) were genotyped in DNA samples from 459 depression cases and 462 individuals with mental resilience (Sample set I). The gender and age characteristics of the individuals with successfully genotyped DNA are given in Table II. SNPs with an indication for allele frequency association with depression in Sample set I (Table III) or in a previously analyzed sample, where winter depression was compared to controls without past or present psychopathology (Table IV), were tested for association to depression in Sample set II where the 459 depression samples were compared with 926 controls (Table III).
First, association between single SNP allele frequency and depression was determined. The SNPs with indication of allelic association with depression (P < 0.05, Table III) were studied with regard to genotype and haplotype distribution difference comparing cases to mental resilience (Sample set I) and to controls (Sample set II) (Tables V and VI). The following suggestive or significant genetic associations with depression were found.
| SNP | Cases %11/12/22 | n | Controls %11/12/22 | n | Test of association, Pa | ||
|---|---|---|---|---|---|---|---|
| Cochran-Armitage trend | Minor allele dominantb | Minor allele recessiveb | |||||
| Sample set I: depression compared to mental resilience | |||||||
| PER2 | |||||||
| rs6431590 | 12/46/42 | 423 | 10/40/50 | 423 | 0.040 | 0.027 | 0.38 |
| rs3739064 | 7.9/38/54 | 432 | 5.3/33/61 | 432 | 0.015 | 0.023 | 0.13 |
| rs10462023 | 13/45/42 | 431 | 16/51/33 | 430 | 0.0045 | 0.0034 | 0.14 |
| rs2304672 | 2.8/20/78 | 432 | 1.4/14/85 | 433 | 0.0054 | 0.0067 | 0.15 |
| NPAS2 | |||||||
| rs1374324 | 12/46/42 | 425 | 17/47/36 | 430 | 0.0071 | 0.042 | 0.014 |
| RORA | |||||||
| rs2028122 | 14/41/45 | 432 | 15/49/36 | 433 | 0.030 | 0.0064 | 0.64 |
| Sample set II: depression compared to controls | |||||||
| ARNTL | |||||||
| rs2290036 | 0.70/17/83 | 429 | 0.58/12/87 | 867 | 0.043 | 0.046 | 1.0 |
| RORA | |||||||
| rs2028122 | 14/41/45 | 432 | 14/49/37 | 864 | 0.033 | 0.0030 | 1.0 |
- Allele 1 is the minor allele.
- a No covariate.
- b Two-sided Fischer's exact test was used if any cell <5.
| Haplotype | Overall frequency | OR [95% CI] | P-value |
|---|---|---|---|
| Sample set I: depression compared to mental resilience | |||
| PER2 | |||
| rs934945–rs6431590–rs3739064 | |||
| CTA | 0.49 | 0.73 [0.60–0.88] | 0.0010 |
| CCG | 0.24 | 1.32 [1.06–1.65] | 0.014 |
| rs10462023–rs2304672 | |||
| CG | 0.10 | 1.58 [1.15–2.15] | 0.0038 |
| TC | 0.38 | 0.76 [0.62–0.92] | 0.0049 |
| NPAS2 | |||
| rs17025078–rs2305160–rs1374324 | |||
| GAA | 0.16 | 0.71 [0.55–0.92] | 0.0092 |
| RORA | |||
| rs2028122–rs4775281 | |||
| AG | 0.36 | 0.82 [0.68–1.00] | 0.051 |
| Sample set II: depression compared to controls | |||
| PER2 | |||
| rs10462023–rs2304672–rs4663302 | |||
| TCC | 0.35 | 0.83 [0.69–0.98] | 0.031 |
| RORA | |||
| rs2028122–rs4775281 | |||
| GT | 0.36 | 0.83 [0.70–0.99] | 0.044 |
| ARNTL | |||
| rs1868049–rs11022778–rs3816358 | |||
| CTA | 0.093 | 1.32 [1.01–1.73] | 0.045 |
- OR, ratio specific haplotype versus all other haplotypes among cases, relative to ratio specific haplotype versus all other haplotypes among controls.
PER2
Block 1 of PER2 (rs934945–rs6431590–rs3739064, Fig. 1) was significantly protective against depression, through haplotype CTA (OR = 0.73, P = 0.001, Pcorr = 0.05), in Sample set I, whereas the CCG haplotype was a suggestive risk haplotype (OR = 1.3, P = 0.01). Likewise, block 2 (rs10462023–rs2304672) showed suggestive protective (TC) and risk (CG) haplotypes (OR = 0.76, P = 0.005, and OR = 1.6, 0.004, respectively). The extended block 2 (rs10462023, rs2304672, rs4663302) protective haplotype TCC was suggestively associated with depression also in Sample set II (OR = 0.83, P = 0.03) (Table VI).
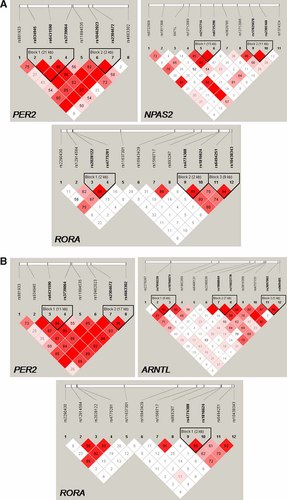
LD structure of the SNPs analyzed in (A) the mental resilience group of Sample set I, and (B) the control group of Sample set II. The numbers in the squares represent the pair-wise D′ value, empty squares stand for D′ = 1. Pink-red color indicates a pair-wise LOD ≥2 with redness proportional to D′. White square indicates LOD < 2. Haplotype blocks are formed if 95% of comparisons are “strong LD” that is the 95% CI of D′ is within [0.7–0.98]. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The haplotype association was supported by suggestive allelic and genotypic association. Four of five SNPs included in the two haplotype blocks were suggestively associated with depression in Sample set I, with the strongest signals for the SNPs in block 2: rs10462023 allele T and rs2304672 allele G showing dominant protective (OR = 0.66, P = 0.003) and risk (OR = 1.6, P = 0.007) effect, respectively (Tables III and V).
NPAS2
The GGA haplotype of rs17025078–rs2305160–rs1374324 was suggestively protective against depression in Sample set I (OR = 0.71, P = 0.009). Likewise, rs1374324 allele A was suggestively protective (P = 0.01).
RORA
The RORA SNP rs2028122 allele A showed a dominant protective effect against depression in both Sample set I (OR = 0.69, P = 0.006, Table III) and Sample set II (OR = 0.70, P = 0.003, Pcorr = 0.03, Table V). The block spanning rs2028122–rs4775281 showed border-line suggestive association with depression, through the protective AG haplotype (OR = 0.82, P = 0.05 and OR = 0.83, P = 0.04 in Sample set I and II, respectively, Table VI). rs2028122 was previously reported to be suggestively associated with winter depression in Swedish cases [Table IV, Sjöholm et al., manuscript].
ARNTL
In Sample set I there was no indication of depression association to any ARNTL SNP. However, since we previously found haplotype CTA of block rs1868049–rs11022778–rs3816358 to be a risk haplotype for winter depression (P = 0.001, Pcorr = 0.05), and border-line single SNP association for rs2290036 and rs3816358 (dominant risk alleles C and A, respectively) to winter depression [Sjöholm et al., manuscript], these associations were tested in Sample set II. Suggestive association was found for both the risk haplotype CTA (P = 0.04) and rs2290036 risk allele C (P = 0.04) with depression (Table VI).
Independence of Environmental Stressors
Negative life events (e.g., serious illness/injury, severe conflicts with spouse or others) and financial strain during the last 12 months increased the risk for depression markedly displaying OR = 9.5 (95% CI: 7.5–12.0) and OR = 7.6 (95% CI: 5.6–10.2) respectively, in combined Sample set I and II. The contribution of the PER2 block 2's genetic variation to depression vulnerability among those without these stressful environmental factors was tested through rs10462023 in Sample set I. Similarly, the association of NPAS2 rs1374324 and RORA rs2028122 to depression vulnerability without exposure to these environmental factors was studied in Sample set I and both Sample sets, respectively (Table VII). ARNTL variation specifically in non-stressor-exposed individuals was not studied since the suggestive association in whole Sample set II was weak. rs10462023 was in complete LD with rs2304672 and hence represented also rs2304672 (Fig. 1). Analysis of the risk effect of the CC risk genotype of PER2 rs10462023 among those with no negative life event showed an OR = 2.0 (P = 0.006), likewise a risk effect was found among those without financial strain showing an OR = 1.6 (P = 0.005). Similarly, a suggested effect of risk genotype GG of rs2028122 was found among those without financial strain with an OR = 1.4 (P = 0.01), whereas no association to depression of this genotype was seen in absence of negative life event. Having NPAS2 rs1374324 risk genotype GG without any of negative life event or financial strain showed only borderline suggestive association to depression, although an estimated OR of 1.7 and above (Table VII).
| Protective genotype | Risk genotype | OR [95% CI], P | |
|---|---|---|---|
| Sample set I: depression compared to mental resilience | |||
| PER2 rs10462023 | TT, TC | CC | |
| Not negative life event | 183 | 110 | |
| Developed depression | 45 | 44 | 2.04 [1.23–3.38], 0.006 |
| Did not develop depression | 138 | 66 | |
| Not financial strain | 453 | 259 | |
| Developed depression | 186 | 135 | 1.56 [1.15–2.12], 0.005 |
| Did not develop depression | 267 | 124 | |
| NPAS2 rs1374324 | AA | GG | |
| Not negative life event | 65 | 176 | |
| Developed depression | 23 | 92 | 2.00 [1.11–3.59], 0.03 |
| Did not develop depression | 42 | 84 | |
| Not financial strain | 167 | 211 | |
| Developed depression | 36 | 131 | 1.73 [1.08–2.76], 0.03 |
| Did not develop depression | 68 | 143 | |
| Sample set I + II: depression versus mental resilience and controls | |||
| RORA rs2028122 | AG, AA | GG | |
| Not negative life event | 659 | 393 | |
| Developed depression | 48 | 41 | 1.48 [0.96–2.27], 0.09 |
| Did not develop depression | 611 | 352 | |
| Not financial strain | 620 | 1,000 | |
| Developed depression | 178 | 143 | 1.38 [1.08–1.76], 0.01 |
| Did not develop depression | 822 | 477 | |
- Total numbers of participants without specified risk factor are in italics.
- OR, ratio number depressed versus non-depressed among those with risk genotype, relative to ratio number depressed versus non-depressed among those with protective genotype.
DISCUSSION
We report that within the core of the circadian system, PER2 genetic variation is associated with depression vulnerability in a Swedish population-based sample, and this genetic risk did not seem to require exposure to potential sleep disturbance factors such as negative life event or financial strain that are known to increase the risk for depression. Polymorphisms in the circadian genes NPAS2, ARNTL, and RORA were suggested to also associate with depression vulnerability. The strategy was to first identify genetic variations in circadian genes indicative of association to depression compared to mental resilience (Sample set I). These variations, as well as variations associated with winter depression [Sjöholm et al., manuscript], were thereafter tested in a second depression-control set (Sample set II). The findings we report for PER2, ARNTL, and RORA are supported by at least two of these three sample sets.
The significant and suggestive PER2 haplotype associations were found for two blocks: block 1 spanning over 21.3 kb (intron 8 to exon 23), and, located 8.2 kb from block 1, block 2 spanning 2.0 kb (exon 2 to intron 3). The block 1 association was found in Sample set I and winter depression versus healthy controls. Block 1 haplotype CTA (rs934945–rs6431590–rs3739064) was protective in Sample set I, whereas TTA was protective in winter depression. This discrepancy may be explained by a 40% lower frequency of the rs934945 T allele in the winter depression sample compared to the depression sample. The association of block 2 (rs10462023–rs2304672) was found in Sample set I and supported in Sample set II. It agrees with our earlier finding of winter depression association with SNP #10870 [Partonen et al., 2007]. The SNP rs2304672 is located only 12 bp upstream of the transcription start site. The PER2 #10870 is located in-between rs10462023 and rs2304672, 1.1 kb from rs2304672 (according to NCBI build 129). Also, rs2304672 risk allele G was nominally associated with bipolar disorder [Kripke et al., 2009], and with morning preferences in healthy volunteers [Carpen et al., 2005]. If the desynchronization of circadian rhythms [Ukai et al., 2007] is linked to the onset of depression in vulnerable individuals, in addition to adverse metabolic and cardiovascular consequences [Scheer et al., 2009], it is highly interesting to note that in mice the Per2 gene is involved in synchronization [Kornmann et al., 2007]. If this role of PER2 holds for humans as well, then PER2 gene variants identified herein may be associated with a reduced synchronicity. To summarize, we show support for association between PER2 and depression for two haplotype blocks, each by data from two case–control sets, and it is further strengthened by earlier reports in other affective disorders.
The NPAS2 block associated with depression in this study is in high LD (D′ > 0.95) with S471L, although having an interdistance of 14.6 kb (according to NCBI build 129). The non-synonymous S471L (rs11541353) SNP has been associated with winter depression in a European Caucasian sample of majority Swedes [Johansson et al., 2003; Partonen et al., 2007].
The ARNTL haplotype CTA of the rs1868049–rs11022778–rs3816358 (block 2) had a weak suggestive protective effect against depression in Sample set II. This same haplotype, CTA was protective against winter depression (Table IV) and a SNP 16.3 kb upstream in block 3 was associated with winter depression in our earlier study [Partonen et al., 2007; The International HapMap Consortium, 2007]. ARNTL has been suggestively associated with bipolar disorder [Mansour et al., 2006; Nievergelt et al., 2006], and an increased ARNTL expression in the brain was reported in a postmortem study of bipolar disorder [Nakatani et al., 2006]. Recently, ARNTL, PER2, and NPAS2 were reported to regulate the transcription of the monoamine oxidase A gene, whose encoded protein inactivates dopamine [Hampp et al., 2008]. Moreover, ARNTL, RORA, and RORB were associated with bipolar disorder in a meta-analysis integrating data from genome-wide association studies and human and animal model expression studies [Ogden et al., 2004; Le-Niculescu et al., 2008]. Herein, the RORA rs2028122 genotype was associated with depression in both Sample set I and II (Table IV).
We analyzed whether the identified main genetic risks were independent of environmental risk factors. The environmental factors studied were limited to negative life events and financial strain the last 12 months. The rationale for this selection was to represent potential sleep disturbing environmental risk factors that may add to genetic risks of rhythm abnormalities in depression vulnerability. Last year negative life events and difficulties were shown to be risk factors for depression in a subpopulation of the PART study [Sjöholm et al., 2009a].
The depression cases, mentally resilient persons and controls are a random selection of an ethnically homogenous population being Swedish nationals and living in Stockholm County at the time of recruitment. Only one-third of the cases reported contact with health care [Forsell, 2006]. Hence, the cases represent a common disease at population level rather than a clinical sample. Less than 10% were of a non-Swedish origin, and the vast majority of those were from a Nordic country. All participants were twice scored for past and present mental illness and present mental well-being. Together, this limits bias due to ethnic variation and dilution of the finding due to disorder heterogeneity among controls. Genetic findings in Sample set I and in winter depression [Sjöholm et al., manuscript] were followed up in Sample set II. A limitation with the study is that the depression cases were the same in the Sample set I and II, hence a similar finding in Sample set II as in Sample set I constitutes a semi-replication. The control individuals in Sample set I were selected for mental resilience, whereas the controls in Sample set II were the remaining healthy individuals in the cohort. A genetic finding in Sample set I not detected in Sample set II may reflect a low effect size in Sample set II, a genetic association to mental resilience rather than to depression in comparison to controls, or a false positive signal in Sample set I.
In conclusion, genetic variation in PER2 associates with depression vulnerability, and that may also be the case for NPAS2, ARNTL and RORA in the Swedish population.
Acknowledgements
The study was supported in part by the Swedish Research Council (2005-6245, 2006-4670), the Stockholm County Council (ALF) and Karolinska Institutet Foundations to Dr. Lavebratt, Dr. Forsell, and Dr. Schalling, and by grants from Academy of Finland (#201097 and #210262) and The Finnish Medical Foundation to Dr. Partonen.




