Impact of Neuritin 1 (NRN1) polymorphisms on fluid intelligence in schizophrenia†‡
Luba Kalaydjieva and Assen Jablensky contributed equally to this work.
How to Cite this Article: Chandler D, Dragović M, Cooper M, Badcock JC, Mullin BH, Faulkner D, Wilson SG, Hallmayer J, Howell S, Rock D, Palmer LJ, Kalaydjieva L, Jablensky A. 2009. Impact of Neuritin 1 (NRN1) Polymorphisms on Fluid Intelligence in Schizophrenia. Am J Med Genet Part B 153B:428–437.
Abstract
Neuritin 1 (NRN1), an activity-regulated gene with multiple roles in neurodevelopment and synaptic plasticity, is located within the 6p24-p25 interval on chromosome 6, previously identified as linked to a subtype of schizophrenia (SZ) characterized by pervasive cognitive deficit (CD). We have tested the effect of NRN1 sequence variation on susceptibility to SZ and on general cognitive ability in patients and non-psychiatric control subjects by re-sequencing the coding regions of NRN1 and its flanking sequences, and genotyping 19 single-nucleotide polymorphisms (SNPs) in 336 SZ patients and 172 healthy control individuals. All participants completed comprehensive neurocognitive assessment, including tests estimating premorbid/prior IQ and current IQ. Logistic regression analyses found no significant association for any of the 19 SNPs with SZ or its CD subtype. However, linear regression analysis gave significant association (P = 0.024 and P = 0.027 after correction for multiple testing) for polymorphisms rs1475157 and rs9405890 with current IQ in the patient group. In SZ, the rs1475157–rs9405890 haplotypes revealed a highly significant association with the abstraction component of current (“fluid”) intelligence (P = 0.0014), and with percentage loss of IQ points between premorbid and current intelligence (P = 0.0041). Results in the control group were not significant after correction. This is the first analysis of association between variation in NRN1 and SZ. The findings suggest a role of NRN1 as a modifier of cognitive functioning in SZ, with implications for future research into the impact of the environment on the development and maintenance of “fluid” intelligence. © 2009 Wiley-Liss, Inc.
INTRODUCTION
The short arm of chromosome 6 has provided some of the best replicated linkage findings in schizophrenia (SZ) genetics [Lewis et al., 2003], with positive findings spread across a very broad interval, raising the possibility that the region harbors more than one susceptibility gene even within ethnically homogeneous samples. While the prospect of multiple genes has been acknowledged, findings of association between DTNBP1 and SZ [Straub et al., 2002] have focused subsequent studies on Dysbindin, now widely regarded as the prime 6p candidate, leaving the issue of other contributing genes unexplored.
In a previous family study [Hallmayer et al., 2005; Jablensky, 2006], with a focus on SZ endophenotypes, we identified two major, only partially overlapping, neurocognitive subtypes, together comprising >90% of the affected subjects: (i) a cognitive deficit (CD) subtype, manifesting with pervasive deficit across multiple cognitive domains, and (ii) a cognitively spared (CS) subtype, with mild or patchy deficits within <1 standard deviation of the performance of healthy controls. Linkage to 6p24-p25 (LOD 3.3), exclusively contributed by the CD subtype, was the most significant finding in a genome-wide scan [Hallmayer et al., 2005]. Using comprehensive HapMap-based association analysis, we excluded DTNBP1 as the gene of interest in our sample [Peters et al., 2008].
The 6p region has also been implicated in normal variation in general cognitive ability, with two independent studies [Posthuma et al., 2005; Dick et al., 2006] reporting linkage to full-scale IQ. General intelligence (g), with its two components: “crystallized” intelligence (cG), the learned verbal knowledge store, and “fluid” intelligence (fG), reflecting problem-solving ability, has long been known to be impaired in the majority of SZ patients [Heinrichs and Zakzanis, 1998; Woodberry et al., 2008]. Early deficits in general cognitive performance, estimated by poor scholastic achievement and below-average IQ scores, have been shown, in prospective and follow-back studies, to be a risk factor for the development of SZ [Zammit et al., 2004; Reichenberg et al., 2006; MacCabe et al., 2008; Woodberry et al., 2008]. Further decline may occur after the onset of clinical illness [Weickert et al., 2000; Badcock et al., 2005; Kremen et al., 2008].
The 6p24-p25 interval contains multiple genes, which satisfy the paramount criterion of being expressed in brain. Among these, Neuritin 1 (NRN1), also known as Candidate Plasticity Gene 15 (CPG15), stands out as an excellent functional candidate. NRN1/CPG15, encoding a small highly conserved protein, attached to the extracellular neuronal membrane by a glycosylphosphatidylinositol link and acting in intercellular signaling with neighboring neurons, was first identified in a screen for activity-regulated genes involved in synaptic plasticity [Nedivi et al., 1993]. In the embryo, the NRN1-encoded protein is expressed in multiple brain regions and functions as a survival factor for neural progenitors and differentiated neurons [Putz et al., 2005]. Later in development, it coordinates the growth of apposing axonal and dendritic arbors and promotes the maturation of glutamatergic synapses by increasing the AMPA/NMDA ratio [Naeve et al., 1997; Nedivi et al., 1998; Cantallops et al., 2000]. Postnatal NRN1 expression, mainly localized to the hippocampus and cortex, increases during critical periods for activity-dependent plasticity and is induced by sensory input and action potential activity [Naeve et al., 1997; Lee and Nedivi, 2002; Fujino et al., 2008]. In addition to synaptic mechanisms involving membrane depolarization, voltage-dependent Ca2+ channels and CaMKII activation, NRN1 expression is promoted by the activity-regulated neurotrophins NT-3 and BDNF. NRN1 is one of the immediate early genes (IEGs) involved in BDNF-induced long-term potentiation (LTP) [Naeve et al., 1997; Lee et al., 2005; Wibrand et al., 2006] and was recently shown to be among the BDNF-regulated genes whose expression increases following chronic antidepressant treatment [Alme et al., 2007].
Neuritin 1's multiple roles in neural development and synaptic plasticity could thus be related to the pathogenesis of CD in SZ, as well as to normal variation in human cognitive ability. To our knowledge, this hypothesis has never been addressed in either the disease or the normal state. Considering the neurobiology of Neuritin 1, we set out to test, in an association study, the effect of NRN1 sequence variation on susceptibility to SZ and on neurocognition as an endophenotype, with a primary focus on the general cognitive ability of affected subjects and controls.
MATERIALS AND METHODS
Subjects
A total of 508 Australian individuals (371 male) of European descent (>75% Anglo-Irish) participated in the study. SZ cases were recruited from consecutive admissions to a psychiatric hospital and community-based mental health services (N = 336, 269 male) and included 93 probands from our previous family study [Hallmayer et al., 2005]. The 172 controls (102 male) were recruited by random sampling from local telephone directories (42%), or among Red Cross blood donors (58%), and screened to exclude history of psychotic illness in themselves or in first-degree relatives, substance dependence, and neurological disease or trauma. Full and accurate information about the study was provided, and written informed consent obtained, from all participants. The study was approved by the Human Research Ethics Committees of the University of Western Australia and the North Metropolitan Area Health Service in Perth, Western Australia.
Diagnostic assessment and assignment to the composite CD/CS endophenotypes involved structured clinical interviews, a neurological examination, and administration of a neurocognitive test battery (Supplementary Table I). SZ cases, meeting DSM-IV and ICD10 criteria, included as nested subphenotype groups 155 CD and 121 CS cases, each characterized further on the quantitative traits of current (“fluid”) and prior/premorbid (“crystallized”) intelligence.
Current intellectual functioning was estimated based on the self-administered Shipley Institute of Living Scale (SILS) test [Zachary, 1986]. The scale consists of a 40-item vocabulary subtest and a 20-item subtest of inductive/abstract reasoning, from which reliable estimates of WAIS-R Full-Scale IQ are derived using conversion tables that take into account the age of the respondent [Zachary et al., 1985]. Prior/premorbid intellectual function level was estimated using the National Adult Reading Test-revised (NART) [Nelson and Willison, 1991]. NART scores were converted to WAIS-R IQ scores, a procedure shown to give high test-retest reliability in SZ patients [Smith et al., 1998].
NRN1 Sequencing Analysis
To discover novel sequence variants in NRN1 and validate the presence of dbSNP registered polymorphisms in our sample, we performed single pass, forward and reverse re-sequencing of the coding regions, ∼50 bp of the flanking intronic sequences and 1,627 bp of the 5′ and 3′ untranslated regions (UTRs) in 47 CD probands from the families that showed linkage to 6p24-p25, three probands from non-linked CS families [Hallmayer et al., 2005], and three controls (PCR and sequencing primers are shown in Supplementary Table II). Applied Biosystems (Foster City, CA) BigDye reagents were used for sequencing and products were run on an ABI 377 DNA Analyzer. Data analysis and comparison to the reference sequence (NC_000006:c5953632-5942232 H. sapiens chromosome 6) was done with Sequencher 4.8© (Gene Codes Corporation, Ann Arbor, MI).
HapMap-Based Selection of Tag SNPs
Coverage of the entire NRN1 genomic sequence and ∼10 kb upstream and downstream was achieved by including additional tag single-nucleotide polymorphisms (tSNPs), selected among HapMap Rel21/phaseII-listed variants with a minor allele frequency (MAF) >10%, using the Haploview pairwise option of Tagger (threshold of r2 > 0.8).
Genotyping and Data Cleaning
The two insertion–deletion (indel) polymorphisms identified by re-sequencing were genotyped with matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) mass spectrometry using single and two-base primer extension reactions. Three SNPs in and around the NRN1 gene, selected from dbSNP (rs107738, rs3749860, and rs582262), were typed with TaqMan assays. Illumina GoldenGate Technology was used for analyzing the 15 HapMap-based tSNPs with DNA samples from CEPH trio 1334 (Coriell Cell Repository, Camden, NJ) as internal controls. The SNP panel included a total of ∼1,100 markers, most of which (with the exception of the 15 NRN1 SNPs, were genotyped for another study. The average genotyping call rate was 99.9%. Eight samples with a call rate <98% were removed. No deviation from Hardy–Weinberg equilibrium (at P > 0.001), analyzed using PLINK [Purcell et al., 2007], was observed in the control samples. The statistical analyses were performed on 500 subjects (171 controls and 329 SZ, including 153 CD and 118 CS) for 19 NRN1 SNPs capturing a total of 31 variants. The genomic positions and MAFs for each of the 19 SNPs are shown in Supplementary Table III.
Statistical Analyses
Using the Genetic Power Calculator [Purcell et al., 2003], power to detect association with the disorder in our principal SZ/control dataset was estimated at 46–74% with homozygote genotype relative risk of 2 at a nominal P = 0.05 for minor allele frequencies ranging from 0.1 to 0.4, and in excess of 89% for homozygote relative risks of 3. Power to detect association with quantitative traits, determined with the Power Sample Size Calculator [Dupont and Plummer, 1990], was 30–99% in the SZ cases at a nominal P = 0.05 for minor allele frequencies from 0.1 to 0.5 and a difference in genotypic means (between those carrying the allele and those without) between 0.2 and 0.5 trait standard deviations.
The analysis of association of each polymorphism with disease outcome in the case/control datasets was performed with chi-square contingency tables and logistic regression (two degrees of freedom tests of genotypic association), implemented in PLINK [Purcell et al., 2007]. On the basis of genotypic association analyses, we explored the data under dominant or recessive models as appropriate, following inspection of the odds ratios (ORs).
Association with premorbid IQ (NART) and current IQ (SILS) scores, equivalent to WAIS-R Full-Scale IQ, was examined separately in the SZ and in the control group using linear regression under additive and genotypic models as implemented in PLINK [Purcell et al., 2007]. Correction for multiple testing was done using the false discovery rate (FDR), which controls the expected proportion of falsely rejected null hypotheses in situations where test statistics are not independent [Benjamini and Hochberg, 1995]. Correction was done in terms of gene-wide significance for the 19 NRN1 SNPs, in accordance with our study hypothesis. Since the CD/CS subgroups of SZ and the premorbid/current IQ measures as their markers are obviously nested features, FDR was estimated based on the truly independent hypotheses, that is, the SZ-control comparisons and each of the 19 SNPs tested.
We used Haploview to determine residual linkage disequilibrium (in the control sample) between SNPs that showed individual evidence of association. Haplotype-based association analyses were performed with SimHap [Carter et al., 2008] under linear regression models. For individuals with ambiguous phase, SimHap imputes haplotypes using an expectation–maximization algorithm and accommodates the imputed haplotypes in the regression analyses by simulation (with 1,000 replicates) using their posterior probabilities [Carter et al., 2008]. We performed a global test, where each haplotype is modeled as an additive effect and examined relative to the most abundant baseline haplotype. Individual haplotypes of interest were examined in haplotype-specific tests.
Post hoc analysis examined the association of the identified haplotypes of interest on the two SILS components (vocabulary and abstract reasoning) and on IQ decline. Decline was measured as the difference between SILS and NART scores, expressed as a percentage of the NART score.
Bioinformatics Analysis
Evolutionary sequence conservation was examined with the ECRbrowser in ECRbase (http://ecrbrowser.dcode.org). Possible transcription factor binding sites (TFBSs) were identified using CONSITE (http://asp.ii.uib.no:8090/cgi-bin/CONSITE/consite/), a web-based software that combines TFBS matrices and evolutionary conservation of binding sites to help reduce false positive results [Sandelin et al., 2004].
RESULTS
NRN1 Sequencing
Analysis of the NRN1 sequences in comparison to the reference NC_000006 identified nine polymorphic positions (Fig. 1), including four SNPs and five indels. Two were known polymorphisms (rs11285278 and rs3834316) and the remaining seven novel. Eleven public database SNPs (rs11752152, rs1115003, rs34544857, rs4141784, rs35678151, rs11550677, rs11550678, rs3173505, rs28618179, rs1052727, rs62408132) were monomorphic in our sample. The coding sequence contained a single rare SNP, c.385C > T (L128L) in exon 3, present in the heterozygous form in one control subject. The remaining polymorphisms (mostly rare, observed in <4% of samples) were located in the UTRs. The four common variants included c.-318delA and c.-214delC (rs11285278) in the 5′UTR, and c.1291delT and c.1295delA, located in a complex repeat sequence in the 3′UTR. Based on their location, the first two indels could be hypothesized to modulate gene expression and were selected for genotyping in the entire sample. The 3′UTR indels were tagged by rs2208870.
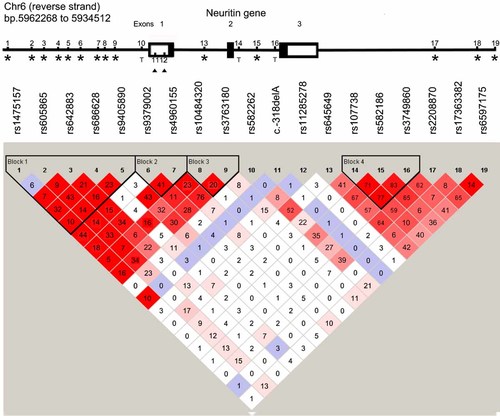
Linkage disequilibrium and genomic location of genetic markers in and around the Neuritin 1 gene. The diagram shows the 19 polymorphisms analyzed in the present study, with their genomic locations and the linkage disequilibrium structure at the Neuritin 1 locus. NRN1 is the only gene within the 20 kb interval shown in the figure. The gene is shown in the direction of transcription on the reverse strand with exons marked as boxes. Coding regions are shown as filled boxes; untranslated regions are unfilled. D′ and r2 values were calculated from genotype data of the control individuals (N = 172) using Haploview 4.1 (http://www.broad.mit.edu/mpg/haploview/). The strength of D′ is indicated by the intensity of red coloring (bright red is D′ = 1) and the values of r2 are in black numbers. Blue squares indicate a D′ of 1 but with a LOD score below 2. Linkage disequilibrium blocks are indicated by solid black lines. Consecutive bold numbers at the top of the LD plot correspond to the numbered black bars on the gene diagram. Polymorphisms identified through sequencing and typed by MALDI-ToF are marked with solid black triangles, polymorphisms chosen from dbSNP and typed by Taqman assays are labeled with “T,” tagging markers selected from HapMap and typed by Illumina GoldenGate Technology are labeled with asterisks. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Association With Dichotomized Schizophrenia Phenotypes
The 19 polymorphisms in and around the NRN1 gene were analyzed for association with the dichotomized phenotypes in the SZ-controls, CD-controls, and CS-controls data sets (detailed results in Supplementary Table IV). No significant results were obtained for SZ and the CD subtype after FDR correction. In the CS/controls data set, rs3763180 showed a nominally significant effect (P = 0.041), non-significant after correction for multiple testing.
Association With General Cognitive Ability
Next we examined the association between NRN1 sequence variation and general cognitive ability in SZ patients and in controls (the complete set of results is contained in Supplementary Table V).
Two variants, rs1475157 and rs9405890 in the NRN1 upstream region, were significantly associated with current intelligence (SILS scores) in affected subjects (Table I and Supplementary Table V). The minor (G) allele of rs1475157 was associated with lower SILS scores: β-coefficient of −4.19 (95% CI −7.09 to −1.29), P = 0.00492 in the allelic test; β-coefficient of 6.20 (95% CI −9.55 to −2.84), P = 0.0013 in the genotypic test and P = 0.0007 for the best-fitting dominant model. The association remained significant after correction for multiple testing (P = 0.047—allelic) and (P = 0.024—genotypic). The minor (G) allele of rs9405890 was associated with higher SILS scores: β-coefficient 4.00 (95% CI 1.56–6.43), P = 0.0014 in the allelic test, β-coefficient of 4.52 (95% CI 1.19–7.86), P = 0.0051 in the genotypic test. Again, the association remained significant after correction for multiple testing (P = 0.027—allelic) and (P = 0.048—genotypic). The two SNPs are in linkage disequilibrium: D′ = 1; r2 = 0.1 (Fig. 1). Analysis of the effect of rs1475157–rs9405890 haplotypes (Table I and Fig. 2) showed that the G-A haplotype (frequency 0.19) was significantly associated with low current IQ (SILS scores), with a regression β-coefficient of −5.85 (−9.31 to −2.39), P = 0.0010. Predictably, the A-G haplotype (frequency 0.28) was associated with higher current IQ: β = 5.04 (1.74–8.33), P = 0.0029.
| Estimated general intelligence | rs1475157 (G allele) | rs9405890 (G allele) | Haplotype-specific association rs1475157–rs9405890 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-value (allelic test) | Corrected P-value | Regression coefficient (95%CI) | P-value (allelic test) | Corrected P-value | Regression coefficient (95%CI) | G-A | A-G | |||
| P-value | Regression coefficient (95%CI) | P-value | Regression coefficient (95%CI) | |||||||
| Premorbid (NART) | 0.230 | 0.511 | −1.24 (−3.27,0.78) | 0.00412 | 0.078 | 2.51 (0.81 to 4.21) | 0.0626 | −2.33 (−4.77, 0.11) | 0.0104 | 3.01 (0.72, 5.30) |
| Current (SILS) | 0.00492 | 0.047 | −4.19 (−7.09,−1.29) | 0.00143 | 0.027 | 4.00 (1.56 to 6.43) | 0.00104 | −5.85 (−9.31, −2.39) | 0.00294 | 5.04 (1.74, 8.33) |
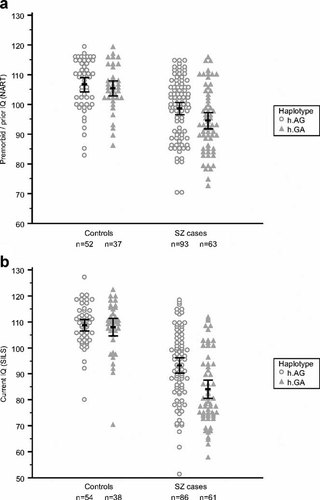
Premorbid/prior IQ and current IQ in schizophrenia cases and normal controls carrying the G-A and A-G NRN1 haplotypes. Individuals with unambiguously defined haplotypes only are included in the scatterplot. Circles and triangles represent individuals. The horizontal bars delineate the mean and 95% confidence intervals. The test scores and effect sizes are shown in Supplementary Table VI.
Analysis of association with premorbid IQ (NART scores) in SZ patients showed no significant main effect of rs1475157 and suggestive evidence for rs9405890: β-coefficient 2.51 (95% CI 0.81–4.21), P = 0.0041 in the allelic test, P = 0.078 after correction. Haplotypic association analysis produced marginally significant results: G-A β = −2.33 (−4.77 to 0.11), P = 0.06; A-G β = 3.01 (0.72–5.30), P = 0.010.
In the group of control individuals, no significant main effect on general cognitive ability (NART or SILS) was observed for any NRN1 polymorphism after correction for multiple testing. In the analysis of rs1475157–rs9405890 haplotype association, we obtained significant results for the G-A haplotype and lower prior IQ (β = −20.24(CI −35.31 to −5.17), P = 0.009 under a recessive model), due to the only two homozygous control individuals having very low NART scores (83 and 85, respectively).
Post Hoc Analyses
The markedly stronger influence of NRN1 variants on current IQ (SILS scores), relative to premorbid IQ (NART scores), led us to conduct further analyses in an attempt to dissect the observed effects. Both NART and SILS assess, in slightly different ways, acquired word knowledge, which tends to remain stable across the life span and is relatively resistant to aging and brain pathology. In addition, SILS contains an abstract reasoning component, which is considerably more difficult to perform than the vocabulary task, and likely to index IQ decrements resulting from neurodegenerative processes. To explore the possibility of a selective influence of NRN1 on “fluid” intelligence, we examined the effect of the rs1475157–rs9405890 haplotypes on the two SILS components, with age as covariate. The results (Table II) revealed a highly significant association with the abstraction component, especially of the GA haplotype which was associated with poorer performance in the abstract reasoning subtest (β = −3.76 (95% CI −6.21 to −1.33), P = 0.0027), but showed more modest association with the verbal SILS component (β = −1.83 (CI −3.16 to −0.50), P = 0.0072). The NART and SILS scores, relative to haplotype, and effect sizes are shown in Supplementary Table VI.
| G-A haplotype | A-G haplotype | |||
|---|---|---|---|---|
| P-value | Regression coefficient (95%CI) | P-value | Regression coefficient (95%CI) | |
| SILS component | ||||
| Vocabulary | 0.0072 | −1.83 (−3.16, −0.50) | 0.0051 | 1.80 (0.55, 3.05) |
| Abstraction | 0.0027 | −3.76 (−6.21, −1.33) | 0.0075 | 3.17 (0.86, 5.48) |
| Change in IQa | 0.0109 | −3.33 (−5.88, −0.79) | 0.0435 | 2.49 (0.08, 4.90) |
- All scores calculated with age as a covariate.
- a Change in IQ measured as the difference between SILS and NART scores, expressed as a percentage of the NART score.
Given the vulnerability of the SILS abstraction component to neurodegeneration, we reasoned that the observed strong association with that component of SILS could reflect a selective NRN1 effect on IQ decline occurring in a subset of patients after the onset of psychotic illness [Weickert et al., 2000; Badcock et al., 2005; Kremen et al., 2008]. Examination of the distribution of haplotype carriers among the intellectually “preserved,” “compromised,” and “deteriorated” groups of patients, as defined by Weickert et al. 2000 and followed up in our sample [Badcock et al., 2005], indicated a clear predominance of the “risk” G-A haplotype in the “deteriorated” group and a reverse, nearly mirror-image, distribution of carriers of the “protective” A-G haplotype (Fig. 3). This observation was supported by a formal analysis of the association between rs1475157–rs9405890 haplotypes and postonset change in IQ (Table II). The G-A haplotype showed a significant association with intellectual decline in patients (measured as the difference between SILS and NART scores, expressed as a percentage of NART): β = −3.33 (CI −5.88 to 0.79), P = 0.0109.
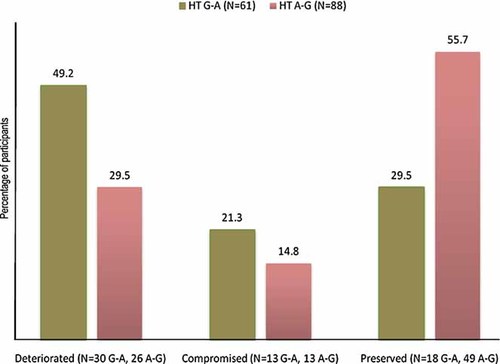
Percentage distribution of G-A and A-G haplotype carriers among schizophrenia patients by degree of change in IQ. Individuals with unambiguously defined haplotypes only are included in the figure. Group definitions are: Deteriorated: patients with a decline in intellectual performance ≥10 points between premorbid (NART) and current (SILS) estimated WAIS-R IQ scores; Compromised: patients with both premorbid and current IQ estimates ≤90 and no evidence of IQ decline >10 points; Preserved: patients with premorbid IQ scores ≥90 and <10 points difference between premorbid and current IQ estimates. Post hoc non-parametric (chi-square test) and parametric (general linear modeling) analyses of the rate of decline as a function of the length of illness (LoI) (≤5 years vs. ≥6 years), age, and haplotype, showed no significant effect of LoI or age. The only factor significantly correlated with intellectual decline was the ratio of haplotype G-A to A-G. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Bioinformatics Analysis
Inspection of the NRN1 sequence in ECRbase showed very high evolutionary conservation of the coding regions and the UTRs in mammals (DNA identity to human sequence of 96.7% in mice to 100% in chimpanzees), with a large proportion also conserved in other species (DNA identity to human sequence of 81.2% in chicken). The high conservation of the coding region fits with the limited variation we found by re-sequencing and indicated any changes in function of this gene between species would probably occur through changes in upstream or downstream regulatory sequences. The sequence surrounding the associated variants (and their tagged SNPs rs9405284 and rs17141536) is conserved in the Rhesus monkey, however, the SNPs themselves are human specific and have not been identified in any other species.
Analysis of the presence of TFBSs and predicted changes introduced by the polymorphic positions showed that the major A allele of rs1475157 is part of a binding site for E4BP4, a suppressor molecule involved in the regulation of circadian rhythm signaling [Mitsui et al., 2001]. This binding site is abolished by the minor “risk” allele of rs1475157. The search identified no relevant changes introduced by the other associated SNP, rs9405890. However, the common C allele of its tagged variant rs9405284 is part of the canonical binding site for another circadian rhythm-related IEG, the transcription factor c-fos as part of the AP-1 transcription complex. While the minor allele of rs9405284 does not abolish the site, it is not part of the canonical sequence recognized by AP-1 and may thus affect binding affinity.
DISCUSSION
Here we report the first analysis of association between Neuritin 1 (NRN1) sequence variation and SZ, and its constituent neurocognitively defined subtypes, as well as with general cognitive ability in patients and controls. NRN1 is a positional and functional candidate gene in the 6p24-p25 region, amply supported by its roles in neural development, activity-dependent synaptic plasticity, and BDNF-induced LTP. This study proceeded from our previous finding of linkage to a narrowly defined region on 6p24-p25 for an endophenotype-anchored subtype of SZ characterized by pervasive CD, with significant contributions of premorbid and current IQ to the cognitive dysfunction trait [Hallmayer et al., 2005]. Added impetus was provided by the observation that while presence of more than one SZ-related gene on the short arm of chromosome 6 had been suggested by a number of studies, the search for candidates within the broad 6p22-p24 linkage region has mainly focused on DTNBP1 in SZ samples, with inconsistent results of replication attempts [Li and He, 2007], including our recent report on lack of association between DTNBP1 sequence variation and susceptibility to either SZ as a clinical phenotype or its neurocognitive subtypes [Peters et al., 2008]. Concomitant support for NRN1 comes from findings of linkage at 6p22-p25 to normal variation in IQ [Posthuma et al., 2005; Dick et al., 2006]. Our study aimed at a comprehensive coverage of sequence variation across the NRN1 gene and its flanking sequences, and analysis of association with: (i) the clinical SZ phenotype, based on DSM-IV/ICD-10 diagnoses; (ii) the neurocognitive composite CD/CS subtypes; and (iii) general intellectual performance. While modest in size relative to current standards, our sample is one of the best characterized for multiple cognitive measures as potential endophenotypes [Jablensky, 2006].
Our analysis did not detect a direct effect of NRN1 polymorphisms on susceptibility to SZ or its dichotomized cognitive subtypes. Since psychometric CD is not part of the definition of SZ in DSM-IV/ICD-10, and the broad diagnostic category harbors considerable heterogeneity [Jablensky, 2006], lack of detectable association between SZ and a gene selectively influencing cognition is not surprising. The absence of an overall association with the CD subtype (which accounted for linkage to 6p24-p25 in our previous study) [Hallmayer et al., 2005] is likely due to the reduced subsample size. At the same time, considering that the CD endophenotype integrates eight cognitive tests (Supplementary Table I), with current IQ having the second largest weight within the composite CD trait, the present finding of a significant association suggests a selective impact of NRN1 on fluid general intelligence. In line with this interpretation, two polymorphisms upstream of NRN1, rs1475157, and rs9405890, showed highly significant main and combined haplotypic effects on current IQ in SZ patients, with a considerably weaker effect on premorbid IQ (Table I). Further analysis revealed a selective effect of these polymorphisms, especially of the G-A haplotype, on fluid intelligence, assessed by the abstract reasoning subtest of SILS (Table II). In agreement with evidence pointing to non-verbal intelligence as particularly vulnerable to disease progression in SZ [Woodberry et al., 2008], we found a predominance of the G-A haplotype in the subset of intellectually deteriorating SZ patients and significant association with the risk of intellectual decline after the onset of manifest psychotic illness. The observed differential effects of NRN1 polymorphisms on cognitive performance in SZ patients support the notion that, although NRN1 may not influence susceptibility to SZ per se, it appears to be a modifier of cognitive functioning in SZ.
The lack of significant results in our small control sample precludes us from drawing conclusions about the effects of NRN1 variants on cognitive performance in the general population, or suggesting that such effects may be more pronounced in the SZ brain. The obvious limitation of the sample size points to the need for replication in normal individuals of different age groups, as well as in subjects affected by dementing disorders.
Our finding of a strong effect of NRN1 on abstraction and inductive reasoning in SZ patients is of particular interest in the broader context of the relationship between crystallized intelligence and fluid intelligence, and the role of the environment in cognitive development [Blair, 2006]. Performance on the abstraction component of SILS presupposes verbal skills, but relies heavily on mental operations requiring flexible analytical reasoning, switching between alternative conceptual strategies, and ability to maintain “online” mental simulation of task outcomes. The neural networks supporting such fluid functions involve distributed but interconnected brain subsystems including prefrontal, temporal, parietal, and occipital cortices, as well as the cerebellum [Gray et al., 2003]. Fluid cognitive skills play a major role throughout mental development and, in fact, may be a key prerequisite for the acquisition of verbal knowledge indexed by crystallized intelligence [Blair, 2006]. While both components of general intelligence are under genetic influence, fluid intelligence is critically linked to dynamic interactions with the environment from the earliest developmental stages [Blair, 2006].
Several functional properties of NRN1 point to a likely role in such interactions. Its expression in the cortex and hippocampus is regulated by sensory input, action potential activity and in response to activity-regulated neurotrophic factors [Naeve et al., 1997; Lee and Nedivi, 2002; Wibrand et al., 2006; Fujino et al., 2008]. The relationship between NRN1 expression, cognitive ability, and circadian rhythms, suggested by our findings, is supported by its role as a light-regulated plasticity gene in the visual cortex [Lee and Nedivi, 2002] and its identification in a microarray analysis as one of the genes upregulated in the cerebellum during wakefulness [Cirelli et al., 2004]. Circadian rhythms and the sleep–wakefulness cycle are increasingly recognized as important factors in memory, learning, and synaptic plasticity [Walker and Stickgold, 2006]. The “risk” allele of rs1475157, associated with poorer performance in the abstraction component of SILS, and IQ decline in SZ patients, disrupts a binding site for the circadian rhythm-related transcription suppressor E4BP4 [Mitsui et al., 2001], possibly uncoupling NRN1 expression from the circadian cycle. The relevance of the hypothesized reduced binding efficiency of c-fos/Jun heterodimers, caused by the minor allele of rs9405284, is unclear. While c-fos is an important neuronal IEG, induced by different forms of activity including wakefulness, NRN1 has not been identified among the genes whose transcription is regulated by c-fos. The functional significance of our associated polymorphisms, as well as the possibility that they flag an unknown functional variant, requires further investigation.
In summary, this study provides the first, tentative evidence that Neuritin 1 (NRN1)—a gene with multiple roles in neurodevelopment and synaptic plasticity—may be critically involved in the neurobiology underlying cognitive dysfunction in SZ.
Acknowledgements
The authors thank the participants in the study; the staff of Graylands Hospital and the North Metropolitan Health Area in Perth, Western Australia, for assistance in patient recruitment; Dr. Kirsten Peters for help with SNP selection; and the Western Australian Genetic Epidemiology Resource for bioinformatics support. The study was supported by grants #404046 and 513874 of the National Health and Medical Research Council of Australia to A. Jablensky and L. Kalaydjieva, with funding contribution from the North Metropolitan Health Area, Perth, Western Australia.




