Promoter polymorphisms which modulate BACE1 expression are associated with sporadic Alzheimer's disease†
How to Cite this Article: Wang S, Jia J. 2010. Promoter Polymorphisms Which Modulate BACE1 Expression Are Associated With Sporadic Alzheimer's Disease. Am J Med Genet Part B 153B:159–166.
Abstract
Beta-site APP-cleaving enzyme 1 (BACE1) gene has been suggested as a candidate gene for Alzheimer's disease (AD). However, little is known regarding the effects of polymorphisms in regulatory sequences of BACE1 on AD susceptibility. To evaluate the relationship between polymorphisms in the BACE1 promoter and sporadic AD (SAD) genetically and functionally, we performed a case-control study (429 cases and 346 controls of Han Chinese descent) and functional characterization of the polymorphisms in vitro using luciferase assay and electrophoretic mobility shift assay (EMSA). Two polymorphisms (−918G/A, rs4938369; −2014T/C, rs3017608) were identified in the BACE1 promoter. The results showed that the −918G/A polymorphism was associated with SAD and the −918GG carriers had a 1.67-fold higher risk for SAD than the carriers with −918AA and GA genotypes (OR = 1.667, 95% CI = 1.087–2.556, P = 0.019). The haplotype −918G/−2014T may be a possible risk factor for SAD (P = 0.016). Luciferase reporter assays showed the −918G allele and its resultant haplotype −918G/−2014T induced an increase of transcriptional activity. A more marked increase in −918G/−2014T transcriptional activity was seen when under hypoxia treatment. EMSA indicated that the −918G allele bound nuclear factors more strongly than −918A allele did. Our findings suggest that the BACE1 promoter polymorphisms which regulate BACE1 expression may contribute to SAD susceptibility. Further independent studies are required to verify our findings. © 2009 Wiley-Liss, Inc.
INTRODUCTION
Alzheimer's disease (AD) is characterized by senile plaques composed of β-amyloid (Aβ) peptide [Masters et al., 1985]. The amyloid hypothesis of AD proposes that this plaque is the primary cause of the disease [Hardy and Selkoe, 2002]. Aβ peptide is generated following the sequential cleavage of the amyloid precursor protein (APP) by β- and γ-secretase [Vassar, 2004]. First, APP is cut by β-secretase to produce the secreted BACE1-cleaved APP ectodomain (APPsβ) and the membrane-bound C-terminal fragment C99. Next, C99 is cleaved by γ-secretase, which releases Aβ. The β-secretase, β-site amyloid precursor protein-cleaving enzyme 1 (BACE1), is the key rate-limiting enzyme for the generation of Aβ [Lin et al., 2000; Cole and Vassar, 2008]. In addition to proteolysis, it was shown in different studies that Aβ production depended on the existence and amount of BACE1 available. For instance, siRNA suppression of BACE1 reduced Aβ production in neurons derived from both wild-type and the Swedish APP mutant transgenic mice [Kao et al., 2004]. BACE1 knockout mice, without developmental deficits, have abolished Aβ generation [McConlogue et al., 2007]. Several studies have shown that both BACE1 mRNA and protein expression are elevated in SAD brains and the elevation of BACE1 enzymatic activity is correlated with brain Aβ1-x where x corresponds to Aβ fragments assayed by the 4G8/6E10 ELISA as previously reported by Li et al. and Aβ1–42 production [Fukumoto et al., 2002; Yang et al., 2003; Li et al., 2004]. It is conceivable that up-regulation of BACE1 expression may contribute to the development of AD. BACE1 regulation is therefore likely to play an important role in the pathogenesis of AD.
Over the past 10 years, most studies have focused on genetic variants in the BACE1-coding region, leading to the suggestion that a silent mutation C/G (rs638405) in exon 5 of BACE1 might be associated with AD risk. However, functional consequence of this polymorphism remains unclear. To date, little reports have paid attention to the gene regulatory region. Two laboratories have recently characterized the genomic organization, structure, and function of the 5′-flanking region of BACE1 [Christensen et al., 2004; Sambamurti et al., 2004]. The BACE1 promoter contains a number of putative transcription factor binding sites (TFBSs) for regulatory transcription factors and BACE1 expression can be induced by a variety of agents including hypoxia [Zhang et al., 2007], heat shock [Lahiri et al., 2006a], disrupted intracellular calcium [Cho et al., 2008], and cytokines [Bourne et al., 2007; Cho et al., 2007]. Variants in the promoters and regulatory regions of certain genes may be relevant in the pathogenesis of AD by altering transcriptional activity [Lahiri et al., 2005; Theuns et al., 2006]. It is reasonable to examine genetic variants in these sequences so as to further explore the pathogenesis of SAD. In the current study we aimed to systematically screen the BACE1 promoter, to detect potential variants, and then to determine whether these variants are associated with SAD, genetically and functionally. The possible association of the variants in BACE1 promoter and the exon 5 C/G polymorphism was also investigated.
MATERIALS AND METHODS
Subjects
Four hundred twenty-nine SAD patients (61% females; mean age at onset = 71.3 years; SD = 7.2; ranging from 55 to 94) and 346 sex- and age-matched healthy controls (63% females; mean age = 72.5 years; SD = 8.1; ranging from 56 to 96) were enrolled in this study. All patients and controls were Han Chinese whose families must have resided for at least three generations in the same area of Northern China (here defined as north to the Yellow River). Patients with SAD were recruited from Xuan Wu Hospital of the Capital Medical University, the Beijing Senile Hospital and several other hospitals in Beijing City. Probable AD was diagnosed clinically according to the criteria of National Institute of Neurological and Communication Disorders and Stroke and the Alzheimer's Disease and Related Disorders Associations (NINCDS–ADRDA) [Mckhann et al., 1984]. None of these patients reported a family history of AD. Control subjects were obtained from the examination center of Xuan Wu Hospital in Beijing who underwent regular health examinations, and were confirmed healthy and neurologically normal by Mini-Mental Status Examination (MMSE), Revised Hasegawa Dementia Scale (HDS-R) and general examinations. Informed consent was obtained for each subject, either directly or from his or her guardian, and the protocol of this study was approved by the Institute Ethical Committee.
Sequencing of the BACE1 Promoter
Systematic screening of BACE1 promoter was performed using standard PCR and direct sequencing in 20 randomly selected controls and 20 SAD patients. Forty samples (20 SAD and 20 controls, 80 alleles) were used to identify the polymorphisms and the approach has >95% power for the detection of polymorphisms that are present at a frequency of ≥5%. Three overlapping primer sets cover the 3393 bp from −2548 to +845 relative to the transcription start site (TSS) as shown in Table I. The sequence of the 3393 bp region is available in http://www.ensembl.org/ (ENSG00000186318 range = chr11:116691568–116694960). TSS is located 691 bp upstream from the translation start site [Christensen et al., 2004].
| Locus | Primer sequence | Length (bp) | Enzyme |
|---|---|---|---|
| Forward: AGCCTCAAGGGCACAAACAG | 1241 | ||
| Reverse: CGGGCTCTTCGTCGGTCT | |||
| Promoter (sequencing) | Forward: CAACAGTTTTTGAATAATGCGTCC | 1437 | |
| Reverse: TCACGAGCAGAGATTGAGGGAG | |||
| Forward: TACAATATGCTGGACACCATTCT | 1167 | ||
| Reverse: TATCTCTACTCTTATCCTACACGG | |||
| −918G/A | Forward: TTTCCTTTCCAAAGCCTGCCT | 162 | Eco130I |
| Reverse: CTCAAGTGATCCGCCCACCT | |||
| −2014T/C | Forward: AGTGAGCTGAGATGAAGCCAGTGC | 159 | Alw44I |
| Exon 5 C/G | Reverse: TCAGAAGTATTCCAATACCACCAGTGTT | ||
| Forward: AGGCACCTATGGTAAACTTGG | 769 | ||
| Reverse: GACTGTTCTAGGCTCAACTTCC | |||
| Promoter (reporter plasmid) | Forward: (GGGGTACC)TACAATATGCTGGACACCATTCT | 3076 | Kpn I |
| Reverse: (GAAGATCT)CTGTGGAGAGCGGTCAGGG | Bgl II |
- Mismatched sites were underlined. Added restriction enzyme recognition sites were shown in brackets.
Genotyping
Polymorphisms in BACE1 promoter were genotyped by restriction enzyme digestion of the PCR products amplified from genomic DNA (Table I). PCR reactions were performed using 50 ng of genomic DNA in 25 µl of reaction mixture consisting of 0.25 µmol of each primer, 0.2 mM dNTPs, 12.5 µl GC buffer (2×) and 1 unit LA Taq DNA polymerase (TaKaRa BIOINC, Shiga Japan). The cycling was performed with an initial denaturation for 5 min at 95°C, followed by 35 cycles at 95°C for 30 sec, annealing for 30 sec, at 72°C for 30 sec with a final extension to 72°C for 7 min. For restriction enzyme digestion, 6 µl of the PCR product was digested by 5 units of the required enzyme in the presence of the accompanying buffer, incubated at 37°C for overnight. Fragments were separated on a 2.5% agarose gel and visualized on an ultraviolet trans-illuminator after ethidium bromide straining. To validate the genotyping results, 10% of the samples were chosen randomly to be re-genotyped by direct DNA sequencing. All results were found to be concordant. The exon 5 polymorphism genotyping was performed by direct sequencing. APOE genotyping was performed according to protocols already described [Hixson and Vernier, 1990].
Construction of Reporter Plasmids
The 3076 bp promoter fragment (from −2548 to +528 relative to the TSS) was amplified from templates corresponding to homozygotes for each allele of both sites of polymorphism using primers (Table I) into which Kpn I and Bgl II restriction sites were introduced. The PCR products from individuals who were homozygous for the −918G/−2014T and −918A/−2014C haplotypes were directionally inserted upstream the firefly luciferase gene in the pGL3-Basic vector (Promega, Madison, WI). Because the −918A/−2014T haplotype was heterozygous in all subjects of our study group, luciferase reporter plasmid containing the −918A/−2014T haplotype was constructed using a QuickChange® Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Negative and positive control were pGL3-Basic (Promega), lacking any promoter sequences, and pGL3-Control (Promega), containing the SV40 promoter and enhancer sequence. A pRL-TK expression plasmid (Promega) containing the herpes simplex virus thymidine kinase promoter upstream of the renilla luciferase gene (Promega) was used as internal reference.
Cell Culture, Transient Transfection, and Treatment of Cells With Different Agents
Human neuroblastoma SH-SY5Y cells and HeLa cells were propagated in RPMI 1640 medium, 15% fetal bovine serum, 200 IU/ml penicillin, and 200 g/ml streptomycin (Invitrogen, Carlsbad, CA). All cells were maintained at 37°C in an incubator containing 5% CO2. For transient transfection, SH-SY5Y and HeLa cells were seeded in 96-well culture dishes at 2 × 104 and 1 × 104 cells/well, respectively, and allowed to recover for 24 hr. Cells were co-transfected with 3 ng of pRL-TK plasmid and 150 ng of either one of the BACE1 promoter constructs or one of the control plasmids, using Lipofectamine 2000 (Invitrogen). Empty pGL3-Basic vector was used as a negative control and pGL3-Control vector as a positive control. Following transfection for 24 hr, Aβ25–35 was added into the normal 1640 medium at 0.015 mM concentration to model pathology of AD; serum deprivation that serum-free 1640 substituted the normal 1640 produced energy starvation and apoptosis. Here we use Aβ25–35, a toxic antigenic fragment which exhibits all the biological activity of the full length Aβ and has been earlier used by other researchers to study the neuronal toxicity and oxidative vulnerability [Jesudason et al., 2008]. Aβ25–35 is a convenient tool for the investigation of neurotoxic mechanisms involved in AD [Frozza et al., 2009]. For hypoxic treatment, Na2S2O4 was added into the normal 1640 medium at 1 mM concentration [Lv et al., 2008]. Transfected cells were treated with these agents for 24 hr before they were lysed and assayed by Dual-Luciferase reporter assay system.
Relative Luciferase Activity Measures
Transfected cells were cultured for 48 hr, washed with 200 µl of phosphate-buffered saline, and lysed with 20 µl passive lysis buffer (Promega). Luciferase activities of firefly (LAF) and luciferase activities of renilla (LAR) were measured sequentially using a Dual-Luciferase reporter assay system (Promega) and a model GloMax™ 96 Microplate Luminometer (Promega). To take account of variations in transfection efficiency for different reporter gene constructs, the relative luciferase activity (RLA) was calculated as: RLA = LAF/LAR.
Electrophoretic Mobility Shift Assay (EMSA)
The double-stranded oligonucleotide probes 5′-CATTTTGGGAGGCCGACGTGGGCGGATCA and 5′-CATTTTGGGAGGCCAACGTGGGCGGATCA (the polymorphic site was both underlined and in bold) corresponding to the −918G or −918A sequence from the BACE1 promoter region were synthesized and end-labeled with biotin. Electrophoretic mobility shift assays were performed by using the LightShift® Chemiluminescent EMSA Kit (Pierce, Rockford, IL). For each binding reaction (10 µl), a total of 100 fmol biotin-labeled probe was combined with 10 µg nuclear extract prepared from SH-SY5Y and HeLa cells, 1 µg poly(dI − dC), and binding buffer. For competition assays, a 50- or 100-fold molar excess of unlabeled −918G or −918A probe was pre-incubated for 20 min at room temperature with nuclear extracts before the addition of the labeled probe. Protein–DNA complexes were analyzed by electrophoresis on non-denaturing 6.5% polyacrylamide gels in 0.25 × TBE and were visualized using chemiluminescent Nucleic Acid Detection Module (Pierce).
Statistical Analysis
Hardy–Weinberg equilibrium was checked using Haploview version 3.32 [Barrett et al., 2005]. Statistical analysis of genotype distributions and allele frequencies was performed by Chi-square test (SPSS for Windows16.0). Logistic regression analysis including three BACE1 polymorphisms, age, gender, and APOE ε4 allele carrier status into one model was performed to obtain adjusted odds ratios (OR) estimates. Interrelations were analyzed by stratification. Linkage disequilibrium (LD) was checked using EH program. LD values (D′ and r2) and estimation of haplotypes were performed in http://analysis.bio-x.cn/myAnalysis.php. The association of haplotypes with AD was assessed by Chi-square test. OR and 95% confidence intervals (95% CIs) were calculated as estimates of the strength of association between genotypes or haplotypes and SAD. The transcriptional activity of the BACE1 promoter was measured by RLA. The two-tailed Student's t-test was used to test the RLA produced by two different promoter constructs, as well as during basal condition and under different agents.
RESULTS
BACE1 Promoter Polymorphisms
The DNA sequencing of BACE1 promoter region in 40 individuals allowed us to identify two single nucleotide polymorphisms (SNPs) which were −918G/A and −2014T/C (rs4938369 and rs3017608, respectively). The genotype and allele distributions of the promoter SNPs and the exon 5 SNP in SAD and control samples were reported in Table II. The distribution of BACE1 genotypes was in Hardy–Weinberg equilibrium in the two groups analyzed. There were significant differences in genotype and allele frequencies for −918G/A between SAD and control (genotype P = 0.011, allele P = 0.004). Stratification according to APOE ε4 allele status revealed the tendency that these differences might be confined to APOE ε4 non-carriers. (Due to the low number of controls in the group of APOE ε4 allele carriers: n = 33, statistical power might not be sufficient and thus these data were not shown). Multivariate logistic regression analysis revealed that an increased risk of SAD was associated with the GG genotypes compared with the GA +AA genotype (OR = 1.667, 95% CI = 1.087–2.556, P = 0.019) (Table III). Our findings suggested that the −918G/A polymorphism in BACE1 promoter might be associated with SAD. We did not find any significant difference of genotype and allele frequencies for −2014T/C between SAD and control before and after they were stratified by APOE ε4. In our sample, the allele and genotype frequencies of BACE1 exon 5 C/G polymorphism were not significantly different between SAD and control even after stratification by APOE ε4 allele status.
| −918 | Genotype | Allele | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | GG (%) | GA (%) | AA (%) | P-value | G (%) | A (%) | P-value | |
| AD | 429 | 285 (66.4) | 134 (31.2) | 10 (2.3) | 0.011 | 704 (82.1) | 154 (17.9) | 0.004 |
| Control | 346 | 195 (56.4) | 136 (39.3) | 15 (4.3) | 526 (76.0) | 166 (24.0) | ||
| −2014 | Genotype | Allele | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | TT (%) | TC (%) | CC (%) | P-value | T (%) | C (%) | P-value | |
| AD | 429 | 256 (59.7) | 157 (36.6) | 16 (3.7) | 0.143 | 669 (78.0) | 189 (22.0) | 0.058 |
| Control | 346 | 183 (52.9) | 145 (41.9) | 18 (5.2) | 511 (73.8) | 181 (26.2) | ||
| Exon 5 | Genotype | Allele | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | CC (%) | CG (%) | GG (%) | P-value | C (%) | G (%) | P-value | |
| AD | 426 | 146 (34.3) | 208 (48.8) | 72 (16.9) | 0.609 | 500 (58.7) | 352 (41.3) | 0.509 |
| Control | 343 | 120 (35.0) | 174 (50.7) | 49 (14.3) | 414 (60.3) | 272 (39.7) | ||
- Frequencies are shown in parentheses. P-values < 0.05 are indicated in bold.
| Wald | P-value | OR (95% CI) | |
|---|---|---|---|
| −918GG vs. GA + AA | 5.494 | 0.019 | 1.667 (1.087–2.556) |
| −2014TT vs. TC + CC | 0.006 | 0.937 | 0.983 (0.642–1.505) |
| Exon 5 CC vs. CG + GG | 0.561 | 0.454 | 0.881 (0.631–1.228) |
- Data were calculated by logistic regression, adjusting for age, gender and APOE ε4 status.
- OR, odds ratio; CI, confidence interval.
- P-value <0.05 is indicated in bold.
LD between alleles at these loci was studied and we found the −918 G/A and the −2014T/C in BACE1 promoter were in LD (D′ = 0.802, r2 = 0.533) (Table IV). The −918G/−2014T haplotype was over-represented in SAD versus control, suggesting it could be a possible risk factor for SAD (OR = 1.320, 95% CI = 1.054–1.652, P = 0.016). There is also a protective −918A/−2014C haplotype which was underrepresented in SAD versus control (OR = 0.725, 95% CI = 0.558–0.942, P = 0.016).
| Haplotype | SAD, n (%) | Control, n (%) | χ2 | P-value | OR (95% CI) |
|---|---|---|---|---|---|
| −918G/−2014T | 647 (75.5) | 484 (70.0) | 5.852 | 0.016 | 1.320 (1.054–1.652) |
| −918G/−2014C | 57 (6.6) | 42 (6.0) | 0.198 | 0.657 | 1.098 (0.727–1.660) |
| −918A/−2014C | 132 (15.4) | 139 (20.1) | 5.807 | 0.016 | 0.725 (0.558–0.942) |
| −918A/−2014T | 22 (2.5) | 27 (3.9) | 2.341 | 0.126 | 0.640 (0.359–1.139) |
- OR, odds ratio; CI, confidence interval.
- P-values <0.05 are indicated in bold.
Transcriptional Activity of BACE1 Promoter Polymorphisms
According to the results of haplotype analysis, we constructed two luciferase reporter plasmids containing the −918G/−2014T haplotype (named pGL-GT) and the −918A/−2014C haplotype (named pGL-AC) whose frequencies were significantly different between SAD and control. In addition, to investigate the effect of the −918G/A site on the transcriptional activity, we also cloned the fragment which was homozygotes of −918A/−2014T (named pGL-AT) into upstream of the luciferase reporter gene (pGL3-Basic). They were transfected into SH-SY5Y cells and HeLa cells which represent central nervous cells and peripheral epithelial cells. As shown in Figure 1, reporter gene assay of transient transfected HeLa cells showed a significant (P < 0.001) 1.5-fold increase in transcriptional activity for pGL-GT compared with pGL-AT and pGL-AC. In SH-SY5Y cells, the more striking 1.7-fold increase in transcriptional activity was observed for pGL-GT versus pGL-AT and pGL-AC. However, there was no significant difference in transcriptional activity between pGL-AT and pGL-AC in either cell line. The relative activities of all these BACE1 promoters were higher in SH-SY5Y cells than in HeLa cells.
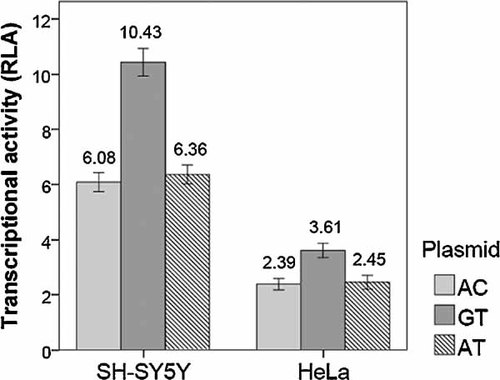
Transcriptional activity of the BACE1 promoters in two cell lines. The figure shows a 1.5 to 1.7-fold increase in transcriptional activity for pGL-GT compared with pGL-AC and AT, and relative activities of BACE1 promoters were obviously higher in SH-SY5Y cells than in HeLa cells. Bars represent firefly/renilla luciferase ratio for the different constructs. RLA, relative luciferase activity.
To further explore gene–environmental interactions, following transfection for 24 hr, cells were exposed to different agents for an additional 24 hr. RLA of different promoter constructs under basal and stimulated condition was indicated in Figure 2. Comparing RLA among different agents, we found that under hypoxia treatment, the BACE1 transcriptional activity of pGL-GT was higher than that during basal condition in both cell lines and the transcriptional activities of pGL-AC and pGL-AT were up-regulated only in SH-SY5Y cells. In SH-SY5Y cells, the increased level of transcriptional activity was greater with the pGL-GT (2.07-fold with hypoxia) than with the pGL-AC and pGL-AT (1.31-fold with hypoxia and 1.28-fold with hypoxia). We failed to detect any difference in transcriptional activities under Aβ25–35 treatment and serum deprivation treatment in either cell line.
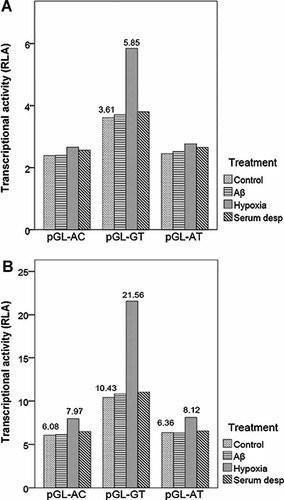
Transcriptional activity of the BACE1 promoters under basal and stimulated condition. Bars represent firefly/renilla luciferase ratio for the different constructs. RLA, relative luciferase activity; (A) HeLa cell; (B) SH-SY5Ycell.
Allele-Specific Transcription-Factor Binding
Using EMSA, we examined whether the −918G/A polymorphic site affecting BACE1 expression interfered with the specific recognition of the BACE1 promoter by nuclear factors extracted from SH-SY5Y cells and HeLa cells. We observed that SH-SY5Y nuclear extracts contained nuclear proteins binding specifically to the −918 site surrounding 29 bp sequence, which resulted in the formation of one major complex (Fig. 3). There were clear differences in binding affinity for the major complex, which preferentially bound to the G allele. Also, pre-incubation with a 100-fold excess of the unlabeled −918G probes completely abolished the formation of the complex that bound to the biotin-labeled −918A probes, while competition with the same amount of the unlabeled −918A probes did not completely inhibit binding of the complex to the −918G allele. We got the same results by using nuclear proteins extracted from HeLa cells. Taken together, these results support the hypothesis of higher binding affinity of this complex for the G allele.
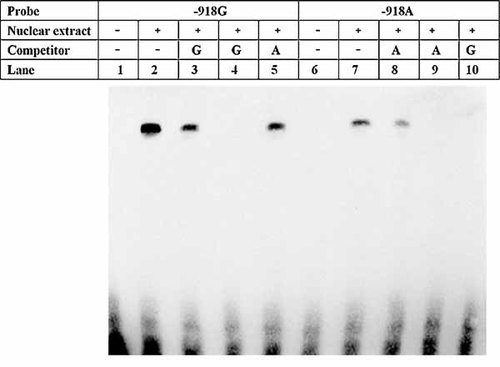
EMSA with biotin-labeled 29-bp oligonucleotides containing the −918G or −918A allele and nuclear extracts from SH-SY5Ycells. Lanes 1 and 6 show the mobilities of the labeled probes without nuclear extracts. Lanes 2 and 7 present the mobilities of the labeled probes with nuclear extracts in the absence of competitor. In competition experiments, 50-fold excess of unlabeled probes (lanes 3 and 8) and 100-fold excess of unlabeled probes(lanes 4 and 9) were added before the addition of the labeled probes; pre-incubation with 100-fold excess of unlabeled probes containing the A allele did not completely block binding of the complex to the-918G allele (lane 5), while 100-fold excess of unlabeled probes containing the G allele completely inhibited the binding of biotin-labeled -918A probes to factors in SH-SY5Y nuclear extract (lane10).
DISCUSSION
Increasingly, reports indicate that BACE1 expression is tightly regulated at both the transcriptional and translational level [Rossner et al., 2006]. Insight into the regulation of BACE1 expression may aid identification of mechanisms that lead to disease, illuminate the role of BACE1 in normal biology, and suggest approaches to inhibit BACE1 therapeutically. Moreover, polymorphic variability within a gene promoter can affect gene expression and has previously been associated with age-related neurodegeneration [Lambert et al., 2001; Belbin et al., 2007; Guyant-Maréchal et al., 2007; Rodríguez-Rodríguez et al., 2007]. Genetic variants in the BACE1 promoter region are therefore appropriate for study to elucidate mechanisms of AD.
In the present study, the promoter region of BACE1 is perhaps firstly screened systematically for variants. We have detected two polymorphisms (−918G/A, −2014T/C) in Chinese Han population and examined the genetic and functional characters of the polymorphisms. The −918G/A polymorphism was significantly associated with SAD susceptibility before and after we stratified gender, age and APOE ε4 via logistic regression. No statistically significant differences in genotype frequencies were observed for −2014T/C between SAD patients and controls. These two polymorphisms were in moderate LD and the −918G/−2014T haplotype conferred a higher risk of developing SAD. Previous genetic association studies of BACE1-coding region have yielded conflicting results. Several reports suggested that an exon 5 C/G polymorphism in BACE1 might be associated with the risk of AD [Kirschling et al., 2003; Shi et al., 2004; Cai et al., 2005; Kan et al., 2005]. In addition, a high-resolution genome screen revealed that certain gene or genes on 11q25, not far from the BACE1, were in linkage with AD [Blacker et al., 2003]. Thus, it can be speculated the variants in BACE1 promoter region may be in LD with the exon 5 polymorphism or other functional variants in another gene nearby, which influences the risk of SAD. To investigate the possible association of the loci in BACE1 promoter and the exon 5 C/G polymorphism, we determined the SNP in the present sample. We failed to find any association between the exon 5 C/G polymorphism and SAD even after statistical adjustment for age, gender and APOE ε4 allele status. Our results were in agreement with two recent studies [Jo et al., 2008; Todd et al., 2008]. LD between the promoter polymorphisms and the exon 5 C/G polymorphism was also analyzed. The degree of LD between the −918G/A and the exon 5 C/G polymorphism was relatively weak (D′ = 0.491, r2 = 0.092). Taken together, these data suggest that the variants in BACE1 promoter may be involved in the SAD risk, independent of the exon 5 C/G polymorphism effect.
To further elucidate the association between these polymorphisms with SAD, we performed functional analyses as well. In the luciferase assay system, we found that the −918G/−2014T haplotype displayed a strikingly higher transcriptional activity compared with the −918A/−2014C haplotype in both the neural and non-neural cell lines. This suggests that the BACE1 promoter with −918G/−2014T may possess higher transcriptional activity and thus over-express BACE1, which plays a pivotal role in the development of AD. Regarding the −918 G/A polymorphism, we detected a significant difference in transcriptional activity between the −918G/−2014T haplotype and the −918A/−2014T haplotype, suggesting the G/A variant might have an effect on BACE1 transcriptional activity. One possible explanation for the functional significance of the −918 G/A polymorphism is that the polymorphism may locate in the key region necessary for promoter activity. There is some evidence obtained from a deletion mapping of the BACE1 regulatory region. The deletion analysis showed that the fragment between −932 and −896, containing the −918 site, harbored an important up-regulatory cis-acting element and deleting the fragment resulted in a drastic loss of BACE1 promoter activity in both neural and non-neural cells [Christensen et al., 2004].
To predict whether the −918G/A polymorphism significantly altered one or more TFBS in BACE1 promoter, sequences of 29 bp length, each corresponding to adjoining sequences of the −918 polymorphic sites, were analyzed by the MatInspector software (http://www.genomatix.de/products/MatInspector/). As a result, the −918A variant was predicted to lose putative binding sites, specifically camp-responsive element binding protein (CREB) and one of two hypoxia inducible factor-1 (HIF-1), while gaining a predicted RXR heterodimer binding site (RXR) when compared to the −918G variant. The BACE1 promoter over 4.1 kb of length contains 20 potential CREB sites which may have implications in APP regulation [Lahiri et al., 2006b]. Mutation of CREB site abolished transcriptional activity of the PSEN-2 promoter [Wang et al., 2006]. The allele-specific effect on BACE1 transcription may be mediated by CREB binding. HIF-1 is the principal molecule regulating oxygen homeostasis [Huang et al., 1999]. When oxygen is in short supply, HIF-1 binds to HRE in promoters or enhancers, thereby activating a broad range of genes involved in angiogenesis, erythropoiesis, cell death, and energy metabolism [Sharp and Bernaudin, 2004]. Loss of function is usually associated with recessive rather than dominant effects. EMSA demonstrated that the −918 G/A polymorphism that influences BACE1 transcriptional activity showed allele-specific binding affinities. It will, however, be necessary to determine whether these particular TFBSs are active elements that, if altered by the polymorphism, increase BACE1 expression.
The sporadic nature of most AD cases strongly argues for an environmental link that may drive AD pathogenesis. To assess whether the specific BACE1 promoter sequence might exert an impact on transcriptional activity in different stress environments and thus altered AD susceptibility, the transfected cells were treated with hypoxia, serum deprivation (low energy stress) and Aβ25–35 (model pathology of AD). We failed to detect any changes in transcriptional activity of these three BACE1 promoters under serum deprivation and Aβ25–35. It is possible that these agents may not alter the BACE1 expression at the transcriptional level. Perhaps the effect of these polymorphisms could not be observed in vitro because of absence of other necessary regulatory elements outside the cloned promoter fragment. Hypoxia treatment markedly increased the three BACE1 promoters' activities and stimulated higher activity with the −918G/−2014T BACE1 promoter than with two other constructs in SH-SY5Y cell, suggesting hypoxia better facilitates over-expression of BACE1 in subjects possessing the −918G/−2014T haplotype than two other haplotype carriers.
In the systematic screen of BACE1 promoter region we identified two polymorphisms, one of which (−918G/A) was associated with increased risk for SAD and showed allele-specific binding of nuclear factors. Both polymorphisms were in LD allowing the identification of a risk haplotype (−918G/−2014T). In short the polymorphisms in BACE1 promoter, which modulate gene expression, may be involved in the onset of SAD.
Acknowledgements
This work was supported by National Key Technology R&D Program in the Eleventh Five-year Plan Period (2006BAI02B01), the National Basic Research 973 Program (2006CB500700), National Natural Science Key Foundation (30830045), Beijing Natural Science Key Foundation (7071004), and Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality. This work was also supported by and partially conducted in the Key Neurodegenerative Lab of Ministry of Education of the People's Republic of China.




