Risk variants in the S100B gene predict elevated S100B serum concentrations in healthy individuals†‡
C. Hohoff and G. Ponath contributed equally to this work.
How to Cite this Article: Hohoff C, Ponath G, Freitag CM, Kästner F, Krakowitzky P, Domschke K, Koelkebeck K, Kipp F, von Eiff C, Deckert J, Rothermundt M. 2010. Risk Variants in the S100B Gene Predict Elevated S100B Serum Concentrations in Healthy Individuals. Am J Med Genet Part B 153B:291–297.
Abstract
Several lines of evidence suggest an important role of the S100B protein and its coding gene in different neuropathological and psychiatric disorders like dementia, bipolar affective disorders and schizophrenia. To clarify whether a direct link exists between gene and gene product, that is, whether S100B variants directly modulate S100B serum concentration, 196 healthy individuals were assessed for S100B serum concentrations and genotyped for five potentially functional S100B SNPs. Functional variants of the serotonergic genes 5-HT1A and 5-HTT possibly modulating S100B serum levels were also studied. Further, publicly available human postmortem gene expression data were re-analyzed to elucidate the impact of S100B, 5-HT1A and 5-HTT SNPs on frontal cortex S100B mRNA expression. Several S100B SNPs, particularly rs9722, and the S100B haplotype T-G-G-A (including rs2186358-rs11542311-rs2300403-rs9722) were associated with elevated S100B serum concentrations (Bonferroni corrected P < 0.05). Of these, rs11542311 was also associated with S100B mRNA expression directly (Bonferroni corrected P = 0.05) and within haplotype G-A-T-C (rs11542311-rs2839356-rs9984765-rs881827; P = 0.004), again with the G-allele increasing S100B expression. Our results suggest an important role of S100B SNPs on S100B serum concentrations and S100B mRNA expression. It hereby links recent evidence for both, the impact of S100B gene variation on various neurological or psychiatric disorders like dementia, bipolar affective disorders and schizophrenia and the strong relation between S100B serum levels and these disorders. © 2009 Wiley-Liss, Inc.
S100B as a member of the S100 calcium-binding protein superfamily is an acidic protein with a molecular weight of 21 kDa and exists as a homodimer composed of two β subunits. It is highly abundant in the human brain, primarily in glia, where it exerts paracrine and autocrine effects on neurons, glia, and microglia. S100B is supposed to play an important role in calcium homeostasis, signal transduction, cell proliferation and differentiation, cellular energy metabolism and cytoskeletal modification [Donato, 2001; Heizmann et al., 2002]. Further, a potential role of S100B-RAGE interactions in S100B neurotrophic and neurotoxic effects is assumed [Donato, 2007]. Therefore, S100B is involved in the regulation of degenerative and regenerative processes in the central nervous system [Rothermundt et al., 2003; Sen and Belli, 2007]. Apart from increased S100B serum levels due to glial cell destruction after brain damage or major inflammation [Lins et al., 2005; Schenatto et al., 2006; Nylén et al., 2008], S100B cerebrospinal fluid and peripheral blood concentrations were shown to be increased in patients suffering from, for example, Alzheimer's disease, mood disorders and schizophrenia [Peskind et al., 2001; Schroeter et al., 2002, 2008; Steiner et al., 2006; Rothermundt et al., 2004a, 2007]. In schizophrenia, elevated S100B levels are associated with negative symptomatology and slower remission upon treatment [Rothermundt et al., 2001, 2004b; Schroeter et al., 2003; Ling et al., 2007].
S100B levels are suggested to be at least partly regulated by the serotonergic system [Rothermundt et al., 2003] which plays an important role in the pathophysiology and treatment of a variety of psychiatric disorders including anxiety, depression and schizophrenia [Terry et al., 2008]. In particular, the serotonin transporter (5-HTT) and 1A-receptor (5-HT1A) are targets of psychopharmacological treatment in schizophrenia and are discussed for their role in neuroprotection [Nichols and Nichols, 2008] particularly in combination with S100B [Eriksen et al., 2002].
The human S100B gene is located on chromosome 21q22.3 [Allore et al., 1988], within or nearby risk regions for familial late-onset Alzheimer's disease [Kehoe et al., 1999], bipolar affective disorder [Cassidy et al., 2007] and Down syndrome [Demirhan and Taştemir, 2003]. Concordantly, association of presumably functional S100B polymorphisms with low cognitive performance and dementia in the elderly [Lambert et al., 2007], bipolar affective disorder with psychosis [Roche et al., 2007] and schizophrenia [Liu et al., 2005] was recently reported. Lambert et al. 2007 identified rs2300403 as risk variant, which possibly favors a splicing event leading to increased expression of S100B2, a recently characterized primate-specific mRNA isoform in the brain. Roche et al. 2007 located rs3788266 within a consensus-binding site for six family transcription factors suggesting that this variant may directly affect S100B gene expression. Liu et al. 2005 reported an association of rs1051169 (renamed rs11542311) and particularly a haplotype spanning rs11542311 and rs9722 with schizophrenia and discussed a genetic predisposition for increased S100B expression as relevant pathophysiological mechanism.
However, it still remains unanswered to date, whether or not S100B gene variants directly modulate S100B serum or cerebrospinal fluid concentration. Alternatively, other genes, gene–gene or gene–environment interactive effects might influence S100B concentration, particularly genes of the serotonergic system.
In the present investigation, S100B serum levels and S100B variants were studied in a sample of healthy individuals to elucidate the influence of S100B, 5-HT1A, and 5-HTT variants on S100B serum levels. Further, publicly available genome-wide human postmortem gene expression data with corresponding genome-wide genotyping data were re-analyzed to study the impact of different S100B, 5-HT1A, and 5-HTT variants on frontal cortex S100B mRNA expression.
The study was carried out according to the Tokyo revision of the Helsinki Declaration and was approved by the Ethical Committee of the University of Muenster. After written informed consent was obtained, 196 healthy blood donors (mean age: 30.3 ± 8.2; f = 72, m = 124) were recruited. In these subjects major psychiatric, neurological or internal disorders were excluded by taking a detailed medical history, performing a physical examination and various laboratory tests for the evaluation of clinical, clinical–chemical and immune-serological parameters.
S100B serum concentrations were determined as described previously [Rothermundt et al., 2004b]. Five S100B SNPs of potential functional relevance were chosen (Table I, Fig. 1) based on previous reports [Liu et al., 2005; Lambert et al., 2007] and in silico analyses using HapMap (http://www.hapmap.org) and UCSC (http://genome.ucsc.edu). They were genotyped by custom TaqMan SNP genotyping assays (Applied Biosystems, Darmstadt, Germany; Supplementary Online Table I) with PCR-preparation on a Genesis Workstation RSP150 (Tecan, Crailsheim, Germany) in 384-well microplates (5 µl-reactions) as recommended by the manufacturer. Amplification and allelic discrimination were performed using ABI Prism 7900SDS with SDS-software v.2.1. For the serotonergic genes the functional variants 5-HT1A-rs6295 (−1019C/G), 5-HTT-LPR and 5-HTT-rs25531 were investigated (Table I) and genotyped according to published protocols [Rothe et al., 2004; Baune et al., 2007]. Genotyping yielded a completion rate of 100% and all genotypes were determined blind to disease status.
| Gene | SNP/variant | Chr | Position | Location | Sample |
|---|---|---|---|---|---|
| 5-HT1A | rs6295 | 5 | 63294321 | Promoter | Serum |
| rs878567 | 5 | 63291747 | 3′-UTR | mRNA | |
| 5-HTT | LPR | 17 | 25588443 to −486 | Promoter | Serum |
| rs25531 | 17 | 25588472 | Promoter | Serum | |
| rs11080122 | 17 | 25571461 | Intron 3 | mRNA | |
| rs8076005 | 17 | 25571336 | Intron 3 | mRNA | |
| rs140701 | 17 | 25562658 | Intron 9 | mRNA | |
| S100B | rs2839364 | 21 | 46850222 | Promoter | Serum |
| rs2186358 | 21 | 46846803 | Intron 1 | Serum | |
| rs11542311 | 21 | 46846658 | Exon 2 | Serum + mRNA | |
| rs2839356 | 21 | 46846572 | Intron 2 | mRNA | |
| rs9984765 | 21 | 46846083 | Intron 2 | mRNA | |
| rs881827 | 21 | 46844296 | Intron 2 | mRNA | |
| rs2239574 | 21 | 46844239 | Intron 2 | mRNA | |
| rs2300403 | 21 | 46845482 | Intron 2 | Serum | |
| rs9722 | 21 | 46843667 | Exon 3 (3′-UTR) | Serum |
- Chr., chromosome.
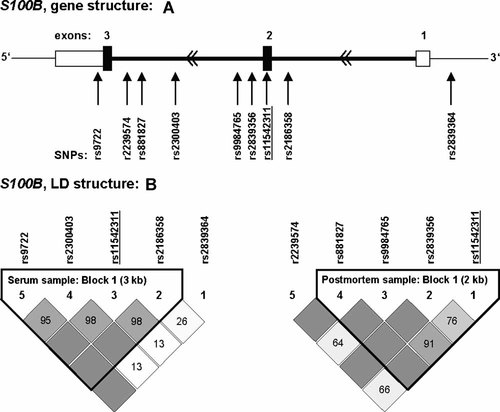
A: Genomic organization of the 6.5-kb S100B gene with translated region spanning exons 2 and 3 (given as black bars) according to the UCSC genome browser (March 2006 assembly). SNP positions relative to the gene are indicated by arrows. B: LD structure of S100B SNPs investigated in the serum sample given on the left and in the postmortem sample given on the right. Underscores mark SNP consistently analyzed in both samples. Shades of gray/numbers in boxes indicate the extent of LD (darker gray/higher numbers = higher D′ values/higher LD) and the resulting haploblock assignment within each sample. Between the samples also high pairwise LD existed (D′ > 0.99), except for SNPs rs9984765-rs2186358 (D′ = 0.66).
Human postmortem S100B mRNA expression was investigated using publicly available datasets [Myers et al., 2007; http://labs.med.miami.edu/myers/LFuN/data.html] comprising 193 unrelated neuropathologically healthy control subjects of European origin. For these controls genome-wide mRNA expression data are provided (assessed by Illumina HumanRefseq-8 Expression BeadChips) as well as SNP genotyping data (assessed by Affymetrix Gene-Chip Human Mapping 500K Arrays). We focused on frontal cortex samples that contain rank-invariant normalized signal intensities for S100B mRNA expression (GI_5454033-S, targeting the S100B-3′-UTR) available for 39 subjects. Genotypes of eight S100B SNPs, two 5-HT1A SNPs, and six 5-HTT SNPs were available. For S100B, these were rs11542311 (also genotyped in the serum sample), rs2839356, rs9984765, rs41387548, rs2239575, rs881827, rs2239574, and rs9983698. Of these, rs41387548 and rs9983698 had to be excluded from analysis due to minor allele frequencies <5% (0.013 and 0.014, respectively) and rs2239575 due to complete linkage disequilibrium (LD) with rs2839356 (D′ = 1.0 and r2 = 1.0). The remaining five SNPs (Table I) possessed high pairwise LD to the S100B SNPs genotyped in the serum sample (D′ = 1.0; except for rs9984765-rs2186358: D′ = 0.66). For 5-HT1A, available SNPs were rs878567 and rs6449693, of which rs6449693 was excluded from analysis due to complete LD with rs878567 (D′ = 1.0, r2 = 1.0). For 5-HTT available SNPs were rs140701, rs8076005, rs11080122, rs2020939, rs2020936, and rs16965623. Of these, rs2020939 had to be excluded from analysis due to deviation from Hardy–Weinberg equilibrium (P = 0.04), rs2020936 due to complete LD with rs8076005 (D′ = 1.0, r2 = 1.0), and rs16965623 due to non-polymorphy. Available 5-HT1A/5-HTT SNPs (Table I) in the postmortem sample are all located within the genes in contrast to the functional variants genotyped in the serum sample which are located in the promoter regions some kilobases apart (5-HT1A-rs6295 distance to rs8785567: 3 kb; 5-HTT-LPR/-rs25531 distance to rs11080122: 17 kb).
Hardy–Weinberg equilibrium and LD of variants were assessed by Haploview v.4.0 [http://www.broad.mit.edu/mpg/haploview; Barrett et al., 2005]. The impact of SNPs and haplotypes on S100B serum levels in the sample of 196 healthy individuals was studied by square root transforming serum values to obtain approximately normally distributed values. Analyses of co-variance, adjusted for age and gender, with the respective geno- or haplotypes as factors of interest were performed by SAS, v.8.2. According to functional studies, 5-HTT-LPR-rs25531 haplotypes were dichotomized into two groups of either higher active (LALA) or lower active alleles [group “rest”: Table II; Lesch et al., 1996; Wendland et al., 2006]. Due to low frequencies (<2%) of minor homozygous genotypes, S100B SNPs rs2839364 and rs9722 were also dichotomized (Table II). Individually reconstructed haplotypes were assigned by PHASE v.2.11 [Stephens and Donnelly, 2003] based on a haplotype reconstruction probability for each individual >0.99. Only haplotypes with frequencies >5% were included in subsequent analyses. The impact of S100B, 5-HT1A, and 5-HTT SNPs on frontal cortex S100B mRNA expression in 39 postmortem controls was re-analyzed using a general linear mixed model. Genotype, postmortem interval and total number of transcripts detected in the sample were assessed as fixed effects; influence of the seven collaborating institutes [Myers et al., 2007] was controlled as random effect. Residuals were normally distributed. Age, gender, and day of expression hybridization were not associated with frontal cortex S100B expression and therefore not controlled for. Due to low frequencies of minor homozygous genotypes S100B SNPs rs9984765, rs881827 and rs2239574 and 5-HT1A/5-HTT SNPs rs878567, rs140701, rs8076005 and rs11080122 were dichotomized (Table III). Haplotypes were individually reconstructed by PHASE with a haplotype reconstruction probability >0.92 for S100B and >0.93 for 5-HTT (three subjects with probabilities <0.9 were excluded).
| Gene | SNP/haplotype | Genotype/allele | N (%) | S100B serum (ng/ml) Mean (SD) | P-valuea |
|---|---|---|---|---|---|
| 5-HT1A | rs6295 | C | 39 (19.9) | 0.061 (0.025) | |
| CG | 111 (56.6) | 0.068 (0.025) | 0.25 | ||
| G | 46 (23.5) | 0.062 (0.021) | |||
| 5-HTT | LPR + rs25531 | LALA | 58 (29.6) | 0.066 (0.025) | 0.82 |
| Restb | 138 (70.4) | 0.065 (0.024) | |||
| S100B | rs2839364 | C | 148 (75.5) | 0.064 (0.024) | 0.30 |
| CT + T | 48 (24.5) | 0.068 (0.024) | |||
| rs2186358 | G | 12 (6.1) | 0.054 (0.012) | ||
| GT | 62 (31.6) | 0.063 (0.026) | 0.22 | ||
| T | 122 (62.3) | 0.067 (0.024) | |||
| rs11542311 | C | 85 (43.4) | 0.062 (0.023) | ||
| CG | 88 (44.9) | 0.070 (0.027) | 0.099 | ||
| G | 23 (11.7) | 0.062 (0.017) | |||
| rs2300403 | A | 86 (43.9) | 0.061 (0.023) | ||
| AG | 86 (43.9) | 0.070 (0.027) | 0.051 | ||
| G | 24 (12.2) | 0.062 (0.016) | |||
| rs9722 | A + AG | 36 (18.4) | 0.076 (0.022) | 0.001 | |
| G | 160 (81.6) | 0.063 (0.024) | |||
| Haplotypesc | T-C-A-G | 172 (43.9) | 0.066 (0.025) | 0.77 | |
| G-G-G-G | 73 (18.6) | 0.062 (0.024) | 0.15 | ||
| T-G-G-A | 35 (8.9) | 0.077 (0.022) | 0.0004 |
- SD, standard deviation.
- a Statistics adjusted for age and gender effects.
- b Group “rest” composed of alleles LALG, LGLG, SASA, SASG, SGSG, LASA, LASG, LGSA, LGSG.
- c Haplotypes consisted of S100B SNPs rs2186358, rs11542311, rs2300403, rs9722 (alleles assessed as present vs. absent). Significant P-values are shown in bold.
| Gene | SNP/haplotype | Genotype | N | S100B expression, meana (SE) | P-valuea |
|---|---|---|---|---|---|
| 5-HT1A | rs878567 | C | 10 | 65.35 (11.20) | 0.38 |
| CT + T | 29 | 75.74 (8.07) | |||
| 5-HTT | rs140701 | C | 16 | 78.43 (8.83) | 0.36 |
| CT + T | 23 | 69.13 (7.61) | |||
| rs8076005 | A | 16 | 81.52 (8.23) | 0.14 | |
| AG + G | 22 | 65.59 (7.35) | |||
| rs11080122 | A + AG | 19 | 65.75 (8.30) | 0.085 | |
| G | 16 | 84.09 (8.66) | |||
| Haplotypesb | C-A-G | 22 | 79.05 (8.22) | 0.21 | |
| T-A-G | 22 | 69.52 (8.36) | 0.26 | ||
| C-G-A | 20 | 66.82 (7.78) | 0.18 | ||
| C | 17 | 63.07 (8.35) | |||
| S100B | rs11542311 | CG | 14 | 70.70 (8.96) | 0.010 |
| G | 8 | 100.06 (10.77) | |||
| rs2839356 | A | 30 | 71.84 (7.21) | 0.66 | |
| AG | 9 | 77.06 (11.61) | |||
| rs9984765 | C + CT | 19 | 84.48 (8.49) | 0.046 | |
| T | 20 | 63.02 (7.97) | |||
| rs881827 | C | 17 | 79.93 (8.80) | 0.23 | |
| CT + T | 22 | 67.95 (7.81) | |||
| rs2239574 | A + AG | 14 | 61.01 (9.07) | 0.083 | |
| G | 23 | 78.55 (7.87) | |||
| Haplotypesb | C-A-T-T | 22 | 67.95 (7.81) | 0.23 | |
| C-A-T-C | 18 | 59.63 (7.67) | 0.008 | ||
| G-A-C-C | 11 | 83.44 (9.74) | 0.18 | ||
| G-A-T-C | 10 | 94.68 (10.21) | 0.004 | ||
| G-G-C-C | 8 | 77.32 (11.97) | 0.66 |
- a Statistics adjusted for postmortem interval and total number of transcripts detected in the sample; SE: standard error.
- b 5-HTT haplotypes comprised SNPs rs140701, rs8076005, and rs11080122; S100B haplotypes SNPs rs11542311, rs2839356, rs9984765, and rs881827 (respective alleles assessed as present vs. absent). Significant P-values are shown in bold.
Significance level was set to 0.05 and results were adjusted for multiple testing by Bonferroni correction reducing the alpha level to 0.005 for analyzing single marker/haplotype effects on S100B serum levels in the serum sample (seven variants plus three haplotypes) and to 0.01 for analyzing S100B SNP effects on S100B gene expression in the postmortem sample (five variants). 5-HT1A/5-HTT postmortem analyses were considered exploratory as data were only available for SNPs within the genes some kilobases apart from the functionally relevant promoter variants genotyped in the serum sample and because no influence of these variants was observed on S100B serum levels. Additionally, due to low sample size, S100B haplotypes were also analyzed in an exploratory fashion only.
In the serum sample genotype frequencies of all variants were within Hardy–Weinberg equilibrium (Pall > 0.05). S100B serum concentration was associated with S100B SNP rs9722 and by trend with rs2300403 and rs11542311 (P < 0.05 and P < 0.1; Table II). Of these, rs9722 showed the strongest effect (Bonferroni corrected P = 0.011) with the rare A-allele associated with elevated S100B level.
LD analysis disclosed one 3-kb haploblock of high LD comprising S100B SNPs rs2186358-rs11542311-rs2300403-rs9722 (Fig. 1), which led to three haplotype alleles T-C-A-G, G-G-G-G, and T-G-G-A. Analyses revealed a strong impact of S100B haplotype T-G-G-A on S100B serum concentration (P < 0.001; Table II). This haplotype included rs2186358 (risk T-allele) and again the three SNPs rs11542311 (risk G-allele), rs2300403 (risk G-allele), and rs9722 (risk A-allele). Association again withstood Bonferroni correction for multiple testing (corrected P = 0.004).
In the postmortem sample all genotype frequencies were within Hardy–Weinberg equilibrium (Pall > 0.05). S100B expression in frontal cortices was associated with S100B SNPs rs11542311 and rs9984765 and by trend with rs2239574 (P < 0.05 and P < 0.1; Table III). Of these, rs11542311 showed the strongest effect on mRNA expression (Bonferroni corrected P = 0.051). Again we identified the G-allele of rs11542311 as risk variant, which was also associated with increased S100B serum levels in this study (haplotype T-G-G-A). Homozygote G-allele carriers were characterized by distinctly higher S100B expression in frontal cortices (about 1.6 times higher) compared to C-allele carriers (Table III).
LD analysis revealed high pairwise LD of S100B SNPs rs11542311, rs2839356, rs9984765, and rs881827, for which a 2-kb haploblock was assigned (Fig. 1) containing haplotypes G-A-C-C, G-G-C-C, C-A-T-C, G-A-T-C, and C-A-T-T. Further one 8-kb block of high LD was detected comprising 5-HTT SNPs rs140701-rs8076005-rs11080122 (D′ = 0.93–1.0), leading to haplotypes C-A-G, C-G-A, and T-A-G. Analyses disclosed S100B haplotypes C-A-T-C and G-A-T-C to be associated with postmortem frontal cortex S100B mRNA expression (P < 0.01; Table III), both differing only in the rs11542311 position. In case of rs11542311-C the haplotype reduced gene expression but in case of rs11542311-G, which also was the risk variant in single marker postmortem analyses as well as haplotype serum analyses, it distinctly increased it.
The present study, to our knowledge, is the first investigating both the serum and genetic level to elucidate the influence of the candidate genes S100B, 5-HT1A and 5-HTT on S100B serum concentration and postmortem frontal cortex S100B mRNA expression. We discovered a strong positive correlation of rs9722-AA + AG and the S100B haplotype T-G-G-A (including rs2186358-rs11542311-rs2300403-rs9722) with S100B serum concentrations, while no such effects of the serotonergic genes 5-HT1A and 5-HTT on S100B were detected. In addition, S100B SNP rs11542311-GG and the corresponding S100B haplotype G-A-T-C (including rs11542311-rs2839356-rs9984765-rs881827) were associated with an increase in S100B mRNA expression in postmortem frontal cortices [re-analyzing public datasets; Myers et al., 2007]. In both samples the G-allele of S100B SNP rs11542311 increased S100B protein or mRNA: In the serum sample within the T-G-G-A haplotype and in the postmortem sample by direct association as well as within the haplotype G-A-T-C. Thus, our study discloses S100B variants to exert strong effects on both the S100B serum and mRNA level. This is in line with studies describing a strong correlation between S100B serum concentrations and S100B cerebrospinal fluid concentrations [e.g. Rothermundt et al., 2004a; Steiner et al., 2006]. The latter might be assumed to directly be modulated by gene expression levels in the brain. Higher levels of S100B mRNA might lead to higher levels of intracellular protein, resulting also in increased S100B cerebrospinal fluid concentrations. However, various regulatory mechanisms might as well interrupt this linear functional relation. MicroRNAs can down-regulate translation by mRNA degradation [Valencia-Sanchez et al., 2006] or differential regulated intracellular secretion mechanisms might reduce the amount of protein secreted in extracellular space [Sen and Belli, 2007], both resulting in decreased cerebrospinal fluid protein concentrations. Further studies are therefore needed to clarify if the strong effects of S100B variants on S100B serum and mRNA level also affect cerebral S100B protein levels. No such effects of the serotonergic genes 5-HT1A and 5-HTT on S100B mRNA expression were observed although S100B levels had been suggested to be at least partly regulated by the serotonergic system, particularly 5-HT1A receptors [Rothermundt et al., 2003].
The very same S100B SNPs rs9722, rs11542311 and rs2300403 were also shown to be associated with schizophrenia in Han Chinese [Liu et al., 2005; rs9722 = V4, rs11542311 = V3] and with low cognitive performance and dementia in the elderly [Lambert et al., 2007; rs2300403], both suggesting clinical relevance of our findings. Furthermore, the observed risk variants might be of functional relevance. SNP rs9722 is located in the 3′ untranslated region (3′-UTR) of S100B (Fig. 1). 3′-UTRs typically contain important regulatory elements described to modulate gene expression, for example, U-rich motifs [Fu et al., 1999], AU-rich elements [Wilusz et al., 2001] or microRNA target sites [Valencia-Sanchez et al., 2006; Vasudevan et al., 2007] which might also apply to rs9722. For rs2300403 Lambert et al. [2007] identified the risk G-allele to increase S100B2 isoform expression in the brain in patients suffering from Alzheimer's disease. For rs11542311 we identified the risk G-allele as strongly associated with increased S100B mRNA expression in postmortem frontal cortices. The risk alleles of all these SNPs suggested or shown to influence gene expression in the brain might be expected to change S100B protein levels also in serum. This should be particularly true when combining them in a haplotype manner. In our study this was indicated by S100B haplotype T-G-G-A, which exerts an even stronger effect on S100B serum level than each of the single variants alone by combining (1) the risk G-allele of rs11542311, (2) the risk G-allele of rs2300403, and (3) the risk A-allele of rs9722. An alternate explanation for the stronger association of the haplotype might be, however, that still another, at present unknown, but functional polymorphism in or around exon 2 is the true causal variant reflected or indirectly captured by the haplotype. Replications of our observations by in vitro studies of S100B gene expression therefore are mandatory. A limitation of this study might be the lack of analysis of additional candidate genes that might also regulate S100B gene expression and the resulting protein levels even though the most promising genes 5-HT1A and 5-HTT did not show any such effect in our serum sample as well as the postmortem sample.
In summary, our results suggest an important role of S100B gene variation on S100B serum concentrations as well as on postmortem frontal cortex S100B mRNA expression. Thus, our study links recent evidence for both, the impact of S100B gene variation on various neurological or psychiatric disorders including schizophrenia and the strong relation between S100B serum concentration and these disorders.
Acknowledgements
We gratefully acknowledge the skilful technical support by Ms. Kathrin Schwarte and Mrs. Christiane Schettler.




