Array comparative genomic hybridization in global developmental delay†‡
Please cite this article as follows: Shevell MI, Bejjani BA, Srour M, Rorem EA, Hall N, Shaffer LG. 2008. Array Comparative Genomic Hybridization in Global Developmental Delay. Am J Med Genet Part B 147B:1101–1108.
Disclosure statement: Drs. Bejjani and Shaffer are co-founders, board members and continue to own an equity interest in Signature Genomic Laboratories LLC. Emily Rorem is an employee of Signature Genomic Laboratories, LLC. The array Comparative Genomic Hybridization studies in this report were undertaken by Signature Genomic Laboratories, LLC. Design and conduct of study, sample collection, management and preparation of manuscript was the responsibility of the corresponding author. Those associated with Signature Genomic Laboratories, LLC (BB, ER, LS) provided assistance in interpretation of results and reviewed and made suggestions to the manuscript prior to its submission.
Abstract
Objective: Array-based comparative genomic hybridization (array CGH) is an emerging technology that allows for the genome-wide detection of DNA copy number changes (CNC) such as deletions or duplications. In this study, array-based CGH was applied to a consecutive series of children with previously undiagnosed non-syndromal global developmental delay (GDD) to assess potential etiologic yield. Methods: The children in this study were drawn from a previously reported consecutive series of children with well-defined GDD. Almost all subjects had undergone prior karyotyping and neuroimaging studies with non-diagnostic results. Array-based CGH was undertaken using the SignatureChip® (1887 BACs representing 622 loci) with abnormalities verified by subsequent FISH analysis and testing of parents to distinguish between pathogenic and familial non-pathogenic variants. Results: On CGH analysis in our study, 6 of 94 children (6.4%) had a causally related pathogenic CNC. Three were sub-telomeric in location. An analysis of a variety of clinical factors revealed that only the presence of minor dysmorphic features (<3) was predictive of etiologic yield on CGH analysis (4/26 vs. 2/68, P = 0.05). Severity of delay was not found to be predictive. Interpretation: In children with non-syndromal GDD, array-based CGH has an etiologic yield of 6.4%. This suggests that this emerging technology may be of diagnostic value when applied subsequent to detailed history, physical examination, and targeted laboratory testing. Array CGH may merit consideration as a first-tier test in the context of a child with unexplained GDD. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Global developmental delay (GDD) is a heterogeneous symptom complex that represents a measurable lag in a young child's attainment of developmental milestones compared to chronologic peers [Shevell, 2005]. Like its presentation and evolution, GDD is etiologically heterogeneous. Extreme variation in reported etiologic yield (from 10% to 80%) [McLaren and Bryson, 1987; Battaglia et al., 1991; Schaefer and Bodensteiner, 1992; First and Palfrey, 1994; Majnemer and Shevell, 1995; Flint and Wilkie, 1996; Shevell et al., 2000a; Srour et al., 2006] reflect variations in sample population characteristics, methods of classification and diagnosis, and the availability of genetic and imaging technology and their rigorous and consistent application to the affected child. Retrospective [Majnemer and Shevell, 1995; Srour et al., 2006] and prospective [Shevell et al., 2000a] studies have highlighted a yield of around 50% for which the top etiologic categories recognized were a mix of intrinsic and extrinsic factors and were in order of decreasing frequency; (i) genetic syndromes/chromosomal anomalies, (ii) intrapartum asphyxia, (iii) cerebral dysgenesis, (iv) severe psychosocial deprivation (i.e., attachment disorder, removal from family home), and (v) ante-natal toxin exposure. The variability in etiologic yield and the spectrum of possible diagnoses challenges the evaluating clinician. To provide guidance, a recent practice parameter of the American Academy of Neurology and Child Neurology Society has put forward an evaluation algorithm for GDD [Shevell et al., 2003]. The American Academy of Pediatrics has also recently formulated an approach to the clinical genetic evaluation of developmental delay/mental retardation (i.e., intellectual disability) [Moeschler and Shevell, 2006]. Both of these position papers have emphasized a systematic individualized approach in which an appreciable yield (i.e., approximately 16% of all those tested) is evident for tests done on a screening basis after an essentially negative history and physical examination. Both position papers have also advocated the future systematic evaluation of the application of novel emerging technologies as possible diagnostic aids in this clinical situation [Shevell et al., 2003; Moeschler and Shevell, 2006].
One such novel technology is micro-array-based comparative genomic hybridization (array CGH) [Bejjani et al., 2005]. This methodology, applied to the entire genome across all chromosomes, enables the detection of abnormal copy numbers of DNA sequences (i.e., copy number changes (CNCs)) that represents sub-microscopic gains and losses of chromosomal segments [Speicher and Higgins, 2007]. Some advantages of array CGH are its ability to detect genetic imbalances not revealed by standard FISH or karyotyping studies and its coverage of sub-telomeric and peri-centromeric regions (i.e., interstitial) [Speicher and Carter, 2005]. Sub-telomeric regions are known to have a high frequency of abnormalities (i.e., 6–8%) in the setting of moderate to severe delay or retardation [Flint et al., 1995]. Furthermore, array CGH provides the opportunity to confirm a suspected clinical diagnosis with a known locus. Early studies with array CGH in the setting of suspected syndromal mental retardation/intellectual disability (i.e., intellectual disability with multiple dysmorphic features and/or major congenital anomalies) have reported promising yields [Menten et al., 2006; Engels et al., 2007].
The current study addresses the question of the possible yield of CGH applied to a consecutive series of children with an as yet non-etiologic diagnosed and non-syndromal GDD. The demonstration of yield and utility of array CGH in such a setting might then warrant its potential application as an investigative test in children with GDD in whom history, physical examination and targeted and prior screening testing have not yet revealed an underlying cause for their developmental disability. This would represent an important modification of present consensus recommendations and clinical practice [Shevell et al., 2003; Moeschler and Shevell, 2006].
METHODS
The subjects of this report were drawn from a consecutive series of children assessed by a single pediatric neurologist over a 10-year inclusive interval (July 1994–June 2004). The process of assembly of this cohort has been described in detail previously [Srour et al., 2006]. From a database containing 5,369 patients seen for varying neurological concerns, 260 children were identified. All these children had a final diagnosis of GDD (defined as a delay in two or more developmental domains of two or more standard deviations on standardized norm-referenced testing) and were below the age of 5 years at the time of initial neurologic assessment. Exclusion criteria were—(i) neurologic assessment not in the context of a general pediatric neurology ambulatory clinic (i.e., patients seen in the context of subspecialty neonatal neurology or neurogenetic clinics were excluded), (ii) prior assessment by another pediatric neurologist (i.e., second opinion), (iii) requested investigations not completed, and (iv) a diagnosis of autism (pervasive developmental disorder) could be made at any point based on the application of DSM-IVR criteria [APA, 2002]. Patients were not selected on the basis of suspicion of a chromosomal abnormality (i.e., presence of dysmorphic features or congenital anomalies). Given a prior study of local referral patterns, it was felt that these 260 children represented an unselected non-biased community-based sample of young children with GDD [Shevell et al., 2001a].
All children had undergone a systematic history and physical examination with subsequent laboratory testing either on a targeted basis, as suggested by findings, or on a screening basis, if no etiologic diagnosis was suspected. Screening tests included karyotyping (standard G-banding initially and high-resolution prophase-banding later), Fragile X molecular testing and neuro-imaging.
As part of the initial study, hospital charts were systematically retrospectively reviewed and variables of interest extracted and recorded. These included age when delay was first suspected, age at initial specialty assessment, family history, prenatal and perinatal history, neonatal history, developmental history and profile, presence of co-existing autistic features or seizures, findings on general and neurologic examinations, presence of microcephaly, results of investigations and etiology (if any) diagnosed. Etiology was defined as per Schaefer and Bodensteiner as; “a specific diagnosis that can be translated into useful clinical information for the family, including providing information about prognosis, recurrence risk, and preferred mode of available therapy” [Schaefer and Bodensteiner, 1992]. Etiologic categories were defined as per Srour et al. [Srour et al., 2006]. Severity of GDD was stratified into mild, moderate, and severe according to the functional age as documented by an evaluating rehabilitation specialist (typically a pediatric occupational therapist) using age-appropriate standardized measures of function and development compared with actual chronological age (i.e., mild: 67–100%, moderate: 33–66%, severe: <33%).
In this original group of 260 children, an etiologic cause as per the above definition was found in 99 (38%) with five etiologic categories (genetic syndromes/chromosomal anomalies, intrapartum asphyxia [invariably including moderate to severe neonatal encephalopathy, acidotic cord pH, and supportive features, including meconium staining, fetal heart rate changes, low Apgar scores beyond 5 min, other organ system involvement and consistent electroencephalographic and imaging changes], cerebral dysgenesis, severe psychosocial deprivation and antenatal toxin exposure) accounting for roughly 75% of diagnoses made [Srour et al., 2006]. Thirteen had abnormal cytogenetic studies. None had undergone sub-telomeric probes. The remaining 161 children in whom no etiology was diagnosed originally were the subject pool for this investigation. Parents were contacted and informed consent was obtained for blood drawing on the undiagnosed children to undergo array CGH.
Microarray Analysis
Array CGH was performed with a targeted bacterial artificial chromosome (BAC) microarray (the SignatureChip®; Signature Genomic Laboratories, Spokane, WA) that was developed for the detection of micro-deletions, micro-duplications, aneuploidy, unbalanced translocations, and sub-telomeric and pericentromeric copy-number alterations (i.e., sub-microscopic CNC) [Hodgson et al., 2001]. Version 4 of the SignatureChip® contains 1,887 BACs representing 622 loci spanning the entire genome. Each locus is represented with a minimum of three overlapping clones. Each locus is flanked by a control contig of three overlapping clones, about 1 Mb on either side. The sub-telomeric and peri-centromeric (i.e., interstitial) regions are represented with at least three overlapping BAC clones, targeted to the unique sequences adjacent to these repetitive regions [Bejjani et al., 2005].
Microarray analysis was performed as described [Bejjani et al., 2005]. Briefly, a dye-reversal strategy was used on two separate microarrays in which 500 ng of both subject and chromosomally normal human control DNAs were labeled (Bio Prime DNA labeling System, Invitrogen, Carlsbad, CA) with Cyanine 3-dCTP (Cy3; Perkin Elmer, Shelton, CT) or Cyanine 5-dCTP (Cy5; Perkin Elmer, Shelton, CT), respectively [Hodgson et al., 2001]. Subject and control DNAs were co-hybridized to one microarray and then oppositely labelled and co-hybridized to a second microarray [Wessendorf et al., 2002]. Images of the hybridized slides were acquired with a GenePix Autoloader or 4000B dual-laser scanner (Axon Instruments, Union City, CA). Spots were analyzed with GenePix Pro 6.0 and Acuity 4.0 imaging and analysis software (Axon Instruments). The mean ratio of fluorescence intensities derived from hybridized subject and control DNA at each test spot on the microarray was calculated and normalized by the mean ratios measured from reference spots on the same slide. The mean ratio of the four normalized spots for each clone was obtained, converted to a log2 scale, and plotted in Microsoft Excel (Redmond, WA).
FISH Analysis
All abnormalities detected by array CGH were confirmed and visualized by metaphase or interphase fluorescence in situ hybridization (FISH) as published using one or more BAC clones determined to be abnormal by array CGH [Shaffer et al., 1994].
In those children in whom array CGH revealed a chromosomal imbalance confirmed on FISH study, parents, and where possible siblings, were then tested in the same way to evaluate whether the imbalance represented a de novo or familial variant. If familial, the cognitive phenotype of the similarly unbalanced parent or sibling was then assessed to evaluate its possible relationship to pathogenesis.
Clinical features of those children with a de novo causative array CGH documented abnormal DNA change and those without were then compared by Fisher's exact test or chi-square analysis to assess if any clinical feature was predictive of positive yield on array CGH testing. A P-value less than or equal to 0.05 was chosen a priori for establishing statistical significance.
RESULTS
Of the 161 children in the original cohort with no etiologic diagnosis for their GDD, 94 (58%) agreed to have blood drawn for array CGH testing. Of the remaining 67, 20 (12%) had been lost to follow-up and 47 (29%) refused participation despite mail and phone contact. A comparison of those participating in the array CGH study and those not participating across a range of clinical variables (i.e., gender, age of initial concern and assessment, frequency of abnormal prenatal history, perinatal or neonatal difficulties, family history, occurrence of seizure disorders, autistic features microcephaly, dysmorphic findings, neurologic abnormalities on examination, or the severity of developmental delay) did not reveal any significant differences. Thus the 94 children participating in array CGH analysis were a representative unbiased sample of the children without an etiology diagnosed for their GDD in the original cohort.
Of the 94 children participating in array CGH analysis, 93 had previously undergone karyotype analysis and 89 had neuro-imaging studies (72 computed tomography, 9 magnetic resonance imaging, and 8 both) with non-diagnostic results reported or determined on subsequent retrospective detailed review.
Initial array CGH analysis revealed an abnormality in 12 subjects (13%). Since some children had more than one array CGH abnormality demonstrated, in total 13 abnormalities were seen (one child had two). Familial testing was then undertaken in all, and six of these were found to be familial, presumably non-pathogenic (i.e., non-etiologic) variants as one cognitively normal parent as determined by interview, educational, and vocational attainment carried the same abnormality as the affected child. The familial variants documented are summarized in Table I.
| Patient PB | Duplication 2q13(2 BAC clones). Proximal gap = 560 Kb. Distal gap = 7.4 Mb | Non-sub-telomeric | Mother samea |
| Patient AR | Deletion 2q13(NPHP1 locus). Proximal gap = 870 Kb. Distal gap = 560 Kb | Non-sub-telomeric | Father samea |
| Patient MG | Duplication 7q11.2 (3 BAC clones). Proximal gap = N/A. Distal gap = 8.12 Mb | Peri-centromeric | Father samea |
| Deletion 9q24.3 (2 BAC clones). Proximal gap = 50 Kb. Distal gap = Mapped | Sub-telomeric | ||
| Patient FW | Duplication 16q24.1 (3 BAC clones). Proximal gap = Mapped. Distal gap = Mapped | Sub-telomeric | Father samea |
| Patient AR-B | Duplication 22 q13.33 (3 BAC clones) Proximal gap = 240 Kb. Distal gap = mapped | Sub-telomeric | Mother samea |
| Patient J-PF | Deletion 18q22.3 (3 BAC clones). Proximal gap = 14.8 Mb. Distal gap = 220 Kb | Sub-telomeric | Father samea |
- a Phenotypically normal (i.e., cognition).
The remaining 6 (6.4%) abnormal array CGH results were felt to be pathogenic and of etiologic significance causally related to the diagnosis of the child's GDD. These are summarized in Table II. In two instances (both males), an abnormality was documented on the X chromosome (Xp22.31 duplication, Xp21 deletion) which was found to be maternally derived from an obviously cognitively intact and normal mother. In one child (male), the array CGH abnormality, a duplication in the SNRPN/UBE3A region on the long arm of chromosome 15, was also found in the mother and younger sister who were both cognitively disabled to a lesser extent than our proband, consistent with the known imprinting of this region [Cook et al., 1997; Schroer et al., 1998]. The cognitively intact mother of one child with an 8p triplication was shown to have a duplication at the same site suggesting a dosage effect for the phenotypic presentation that has not been previously reported. The remaining three children with array CGH abnormalities had on family testing a unique CNC demonstrated. Of the six children with an array CGH abnormality demonstrated, all had undergone normal neuro-imaging testing (four computed tomography, one magnetic resonance imaging, one both) and all had undergone standard karyotyping. Of the six chromosomal abnormalities demonstrated in the six children, three were sub-telomeric in chromosomal location (1p deletion, 8p triplication, Xp22 duplication) and the remainder interstitial in location. Examples of abnormalities documented are shown in Figures 1 and 2.
| Patient A-AA | Deletion Xp21 (3 BAC clones) Proximal gap = Mapped. Distal gap = mapped | Non-sub-telomeric | Mother samea |
| Patient DL | Duplication Xp22.31 (12 BAC clones) Proximal gap = Mapped. Distal gap = Mapped | Sub-telomeric | Mother and sister samea |
| Patient MM | Deletion 1p36.33(17 BAC clones) Proximal gap = 410 Kb. Distal gap = N/A | Sub-telomeric | De novo |
| Patient NM | Deletion 2p11.2(2 BAC clones). Proximal gap = N/A. Distal gap = 390 Kb | Non-sub-telomeric | De novo |
| Patient SG | Triplication 8p23.3(5 BAC clones). Proximal gap 50 Kb. Distal gap = 240 Kb | Sub-telomeric | Mother duplicationa |
| Patient NJHZ | Duplication 15q11.12(8 BAC clones- SNRPN/UBE3A locus) Proximal gap = N/A. Distal gap = 4.1 Mb | Non-sub-telomeric | Mother/Sister sameb |
- Non-sub-telomeric = interstitial.
- a Phenotypically normal.
- b Cognitive disability (less severe).
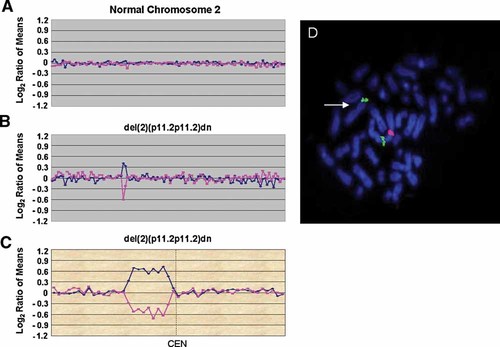
Identification of a de novo pericentromeric deletion by array CGH. Array CGH data are displayed distal to proximal on the left side of the plot for the short arm clones of each chromosome and proximal to distal on the right for the long arm clones of each chromosome. The blue line is a plot of the data from the first array CGH experiment (reference AF647/patient AF555). The pink line is a plot of the data from the second array CGH experiment in which the dyes have been reversed (patient AF647/reference AF555). A: SignatureChip® chromosome 2 plot showing a normal chromosome 2. B: SignatureChip® chromosome 2 plot from Patient NM showing a del(2)(p11.2p11.2)dn. This plot shows a deletion of two BAC clones from the pericentromeric region of 2p. C: Signature MarkerChip™ chromosome 2 plot from Patient NM showing a del(2)(p11.2p11.2)dn. This plot shows a deletion of nine BAC clones spanning ∼1.6 Mb. D: Fluorescence in situ hybridization (FISH) analysis using BAC clone RP11-829C6 (red) from the 2p pericentromeric region showing a deletion at 2p11.2 (arrow). The 2p subtelomere probe RP11-356M6 (green) was used as a control.
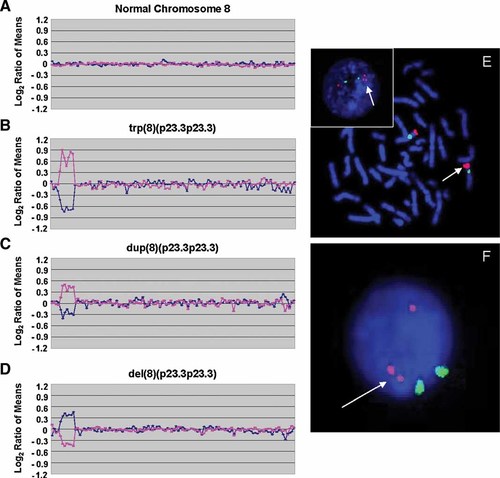
Identification of a triplication of 8p23.3 by array CGH. Array CGH data are displayed distal to proximal on the left side of the plot for the short arm clones of each chromosome and proximal to distal on the right for the long arm clones of each chromosome. The blue line is a plot of the data from the first array CGH experiment (reference AF647/patient AF555). The pink line is a plot of the data from the second array CGH experiment in which the dyes have been reversed (patient AF647/reference AF555). A: SignatureChip® chromosome 8 plot showing a normal chromosome 8. B: SignatureChip® chromosome 8 plot from Patient SG showing a trp(8)(p23.3p23.3). This plot shows a triplication of seven BAC clones spanning ∼1.0 Mb. C: SignatureChip® chromosome 8 plot from the mother of Patient SG showing a dup(8)(p23.3p23.3). This plot shows a duplication of the same seven BAC clones spanning ∼1.0 Mb as in the child. D: SignatureChip® chromosome 8 plot of hybridization of the mother's DNA hybridized against the child's DNA (mother/child). The mother has one less copy of 8p23.3 compared to the child (2 copies vs. 3 copies). E: Fluorescence in situ hybridization (FISH) analysis of Patient SG using BAC clone CTD-2387E16 (red) from the 8p23.3 region showing a triplication at 8p23.3 (arrows). The inset shows an interphase cell demonstrating the triplication. The chromosome 8 centromere control probe, D8Z2 (green) showed a normal hybridization pattern. F: Interphase FISH analysis of the mother of Patient SG using BAC clone CTD-2387E16 (red) from the 8p23.3 region showing a duplicated signal. The chromosome 8 centromere control probe, D8Z2 (green) showed a normal hybridization pattern.
As a group the six children with a presumably pathogenic CNC array CGH abnormality had the following clinical features; five were male, none had an abnormal prenatal history, none had any evident perinatal difficulties, one had neonatal difficulties, three had a family history of an affected developmentally impaired relative, one had co-existing seizures, three had a single autistic feature (i.e., poor eye contact) insufficient for the diagnosis of an autistic spectrum disorder as per standardized formal diagnostic assessment, one was microcephalic, two had abnormal objective findings on neurologic examination, and four were mildly dysmorphic (less than three dysmorphic features insufficient to suspect a specific syndromal diagnosis or chromosomal abnormality). With respect to delay severity, three had moderate GDD and three had severe GDD. The individual features of each child with an array CGH abnormality is tabulated and summarized in Table III.
| Name | Gender | Prenatal Hx | Perinatal Hx | Neonatal Hx | Family Hx | Micro | Seizures | Neuro findings | Autistic | Dysmorphic findings | Severity delay |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A-AA | M | N | N | N | Yes; Brother-hemiplegic CP | No | No | No | No | Yes; Hyperextensible fingers; Widely spaced teeth | Severe |
| DL | M | N | N | N | No | No | No | No | Yes | No | Severe |
| MM | F | N | N | N | Yes; Pat GF-congenital-visual impairment | Yes | No | Yes; Hypotonia | No | Yes; Frontal bossing; Low set ears | Moderate |
| NM | M | N | N | Abnormal jaundice gavage feeding | No | No | Yes; febrile seizure (age 2) | Yes; Hypotonic | No | Yes; Prominent ears; Hypotelorism | Moderate |
| SG | M | N | N | N | No | No | No | No | Yes | Yes; Small genitalia; Puffy hands and feet | Moderate |
| NJHZ | M | N | N | N | Yes; mother/sister cognitively disabled | No | No | No | Yes | No | Severe |
A variety of clinical features were assessed regarding their predictive capability of abnormal array CGH results. Only the presence of some minor dysmorphic features (<3 in total) was found to be a statistically significant predictor. Gender, family history, severity of delay, prenatal/perinatal/neonatal difficulties, co-existing seizures or autistic features, microcephaly, and an abnormal neurologic finding on examination were not found to be predictive of a CNC array CGH abnormality.
DISCUSSION
Improvement in etiologic yield in the setting of GDD/mental retardation reflects the application of emerging genetic and imaging technologies over the past generation to this clinical population [Shevell, 2006]. Prior published studies on array CGH have suggested a potential diagnostic role for this means of detecting CNC across the genome that represent potentially causal sub-microscopic segmental aneusomies (i.e., imbalances). However, populations studied previously appeared to have been pre-selected to heighten the etiologic yield.
Toruner et al. (2007) in a preliminary study of 15 children with mental retardation and a known previously documented karyotype/FISH abnormality demonstrated a 100% concordance rate for array CGH. In a group of 30 individuals with mental retardation and dysmorphic features, Miyake et al. (2006) found five array CGH abnormalities of which two were sub-telomeric in location.
Six studies have reported array CGH yields ranging from 9.7% to 15% in samples of individuals with mental retardation/intellectual disability and a suspected, though not yet proven by routine karyotyping (typically high-resolution G-banding), underlying chromosomal abnormality [Vissers et al., 2003; Shaw-Smith et al., 2004; Schoumans et al., 2005; de Vries et al., 2005; Menten et al., 2006; Engels et al., 2007]. All of these cohorts have significant dysmorphic features and/or one or more associated major congenital anomalies [Bar-Shira et al., 2006].
In a recent study, Engels and colleagues analyzed 60 individuals with mental retardation and congenital anomalies [Engels et al., 2007]. Careful reading of their subjects' characteristics reveals significant associated neurologic compromise with 12 of 60 (20%) having abnormalities already on neuro-imaging, 15 of 60 (25%) a co-existing seizure disorder, and 31 of 60 (52%) with prominent neurologic findings on examination (i.e., ataxia, dystonia). The sample also had a median de Vries score of 3, highlighting substantial associated dysmorphic features. In this study, six individuals (10%) were found to have an array CGH abnormality. The authors note that all of these individuals had facial dysmorphology and the yield amongst the subset having a documented brain abnormality on neuro-imaging was twice that of those with normal neuro-imaging results.
Thus for the most part, an appreciable yield for array CGH has thus far been documented in a pre-selected population where clinical suspicion for a chromosomal abnormality exists and there is a reasonable a priori expectation for this appreciable yield. In a relatively unselected population, de Vries and associates' found 10 de novo segmental aneusomies in 100 patients with mental retardation (10% yield) [de Vries et al., 2005]. Severity of the documented mental retardation or any observed dysmorphic features was not found to be predictive of an array CGH abnormality in this cohort. Some pre-selection of the original cohort with respect to dysmorphology and prior growth deficiencies was noted by the authors of this report leading them to suggest that the array CGH yield found was likely an over-estimate (see below).
Utilizing the same targeted genomic micro-array analysis employed in this study, clinically relevant genomic alterations were documented in 84 (5.6%) of a consecutive series of 1,500 clinical cases sent for analysis for a variety of developmental problems [Shaffer et al., 2006]. As these cases were referred from a number of referral sources, information on their clinical characteristics could not be reliably ascertained for detailed analysis. Approximately half of the cases in which relevant genomic alterations were documented by array CGH had undergone prior non-diagnostic genetic testing.
Our study consists of a non-preselected, well characterized, consecutive series of children in whom detailed assessment, including sub-specialty evaluation, routine karyotyping, FMR1 molecular genotyping and neuro-imaging had not determined an underlying etiology. In this cohort, there was no enhanced a priori suspicion of a possible not yet recognized syndromal or chromosomal abnormality. All of these children had been seen originally as pre-schoolers and reflect the typical pattern of GDD encountered in ambulatory practice. In this cohort, we determined a 6.4% yield on array CGH testing. This figure is similar to the yield estimated by de Vries et al. (2005) to be expected in an unselected series of patients based on a detailed analysis of their sample (7.3%; 95% CI [4.2%–10.3%]). Even if all those who did not participate in our study were in fact normal on array-CGH testing (i.e., a worst case scenario) the rate of diagnosis would be 6/161 or 3.7%. Both of these yields are well in excess of the 1% threshold selected by the AAN/CNS Practice Parameter sub-committee as a clinically meaningful cut-off for consideration of a particular diagnostic test in the setting of GDD [Shevell et al., 2003].
For the most part, in our study clinical features of a child's GDD were not found to be predictive of array CGH yield. The only exception to this was the presence of minor dysmorphic features. Yield in the presence of minor dysmorphic features was 4 of 26 individuals (15.4%), whereas in the absence of any dysmorphic features yield, though significantly lower at 2 of 68 (2.9%), was still well above the 1% threshold and in the range expected for cytogenetic testing and Fragile X molecular geneotyping [Shevell et al., 2003]. As in de Vries' study [de Vries et al., 2005] and in recent prospective [Shevell et al., 2000a] and retrospective [Srour et al., 2006] studies of etiologic yield and GDD as a whole, the severity of the observed delay was not predictive of enhanced yield on etiology testing. This suggests that the vigor of the diagnostic work-up for GDD or mental retardation should be equally applied across the spectrum of severity. This is consistent with recent consensus statements [Shevell et al., 2003; Moeschler and Shevell, 2006].
Our study cohort is also quite distinct from that of Engels and colleagues [Engels et al., 2007]. In our study, none of the cohort had neuro-imaging abnormalities, 4 of 94 (4.2%) had co-existing seizures, and 11 of 94 (12%) had neurologic findings on examination compared to 20%, 25%, and 52% for these attributes, respectively, in Engels' study. In an Editorial accompanying the publications of Engels et al. paper, Speicher and Higgins correctly concluded that array CGH represents an important advance for the genetic evaluation of individuals with syndromal mental retardation [Speicher and Higgins, 2007]. However they highlight that “this technology will have limited utility in the evaluation of individuals with non-syndromal mental retardation” and that these patients “are in need for innovative genetic diagnostic tools to aid in their evaluation” [Speicher and Higgins, 2007]. The clinical characteristics of our study's cohort and our array CGH yield would suggest otherwise.
Microscopic cytogenetic analysis using presently available standardized techniques has a resolution of 5–10 Mb, whereas array CGH resolutions can be 1 Mb or less [Solinas-Toldo et al., 1997]. An abnormal array CGH result requires FISH study for confirmation and parental sampling to rule out familial sub-microscopic variation [Pinkel et al., 1998]. Array CGH cannot yet pick-up trinucleotide repeats that characterize Fragile X. One wonders if array CGH may eventually replace routine cytogenetic analysis as it possesses the capability of detecting both microscopic and sub-microscopic (including sub-telomeric) aneusomies/CNC as well as confirming a suspected genetic syndrome now confirmed by targeted FISH testing (e.g., Prader–Willi syndrome) [Speicher and Higgins, 2007]. Extrapolating our results from the present study to our original cohort of 260 children with GDD [Srour et al., 2006], array CGH would have detected 17 chromosomal abnormalities in the cohort as a whole representing a 7.7% yield (n.b; diagnosis subsequent to history and physical examination precluded the need for karyotyping in 39 [15%] of the original 260 children). Similarly, using array CGH as a screening test together with FMR1 genotyping and neuro-imaging would have increased the yield from 18 of 113 (16%) to 25 of 113 (22%) subsequent to a non-suggestive (i.e., non-diagnostic) history and physical examination. The utility of replacing routine karyotyping with array CGH remains to be evaluated by future systematic study. Ongoing technological advances provide an increasing capability of array CGH to detect smaller CNC suggesting the potential for an even more enhanced yield. Also remaining speculative is the possible role of array CGH testing in other neurodevelopmental disabilities, especially those for which an underlying etiology is rarely found, specifically the autistic spectrum disorders [Shevell et al., 2001b; Sebat et al., 2007] and developmental language impairment [Shevell et al., 2000b].
Array CGH also provides the benefit of identifying possible genes involved in mental development, specifically the mechanisms of learning, memory, and adaptation (i.e., plasticity) which are aberrant in developmental disability and mental retardation. Array CGH provides a mechanism of rapidly and precisely identifying and mapping possible candidate genes that will increase our knowledge regarding single gene defects underlying developmental delay/mental retardation. It has been suggested by others that establishing and utilizing standardized reference databases that will catalogue both normal and disease-causing (i.e., pathogenic) CNC will both simplify strategies to establish causality in the future and provide for more robust genotype–phenotype correlations [Speicher and Higgins, 2007].
While remaining to be validated in other cohorts, it appears that based on our study and those of others, array CGH will improve etiologic yield across the spectrum of individuals with GDD or mental retardation, either clinically suspected as syndromal or not a priori thought to be syndromal in origin. Utilizing array CGH appears to improve the robustness, fidelity, and thoroughness of the clinical evaluation of this population. Through its systematic use, uncovering why a child is developmentally or cognitively disabled can be more often expected by the evaluating specialist. The recognition of single genes associated with aberrant development will also serve to further our understanding of the genetic mechanisms by which cognition proceeds both in the normal and abnormal setting.
Acknowledgements
MS is grateful for the support of the Montreal Children's Hospital Foundation during the writing of this manuscript. Alba Rinaldi provided the necessary secretarial assistance.