Family-based association analysis of a statistically derived quantitative traits for ADHD reveal an association in DRD4 with inattentive symptoms in ADHD individuals†
Please cite this article as follows: Lasky-Su J, Lange C, Biederman J, Tsuang M, Doyle AE, Smoller JW, Laird N, Faraone S. 2007. Family-Based Association Analysis of a Statistically Derived Quantitative Traits for ADHD Reveal an Association in DRD4 With Inattentive Symptoms in ADHD Individuals. Am J Med Genet Part B 147B:100–106.
Abstract
The objective of this study was to determine whether single nucleotide polymorphisms (SNPs) within candidate genes for ADHD are associated with quantitative phenotypes generated from inattentive and hyperactive-impulsive symptoms. One hundred forty-three SNPs were genotyped in and around five ADHD candidate genes. A highly heritable quantitative phenotype was generated at each SNP by weighting inattentive and hyperactive-impulsive symptoms. Once these phenotypes were generated, a screening procedure was used to select and test the five SNP/phenotype combinations with the greatest power to detect an association for each candidate gene. Adjacent SNPs in the promoter region of DRD4, hCV26775267 and hCV26775266, were associated with the quantitative phenotypes generated from the ADHD symptoms (corrected P-values = 0.012 for both SNPs). The correlations between the ADHD symptoms and quantitative phenotype revealed that inattentive symptoms had a strong influence on the generated phenotype. Subsequent family-based association test-principal components (FBAT-PC) analyses using inattentive symptoms only also had significant associations. SNPs in the promoter region of DRD4 are associated with the phenotypes generated from ADHD symptoms. The strong correlation of the inattentive symptoms with these quantitative phenotypes and the subsequent FBAT-PC analyses suggest this region is primarily associated with inattentive symptoms. This analysis adds to previous findings by suggesting that variants at these loci may be specifically associated with inattentive symptoms. © 2007 Wiley-Liss, Inc.
INTRODUCTION
In 1994, the American Psychiatric Association defined three subtypes of ADHD in the Diagnostic and Statistical Manual, version four (DSM-IV) [American Psychiatric Association, 1994]: inattentive, hyperactive-impulsive, and combined. Impairment in inattentiveness or hyperactivity and impulsivity must begin to manifest by the age of seven and persist for at last 6 months to be diagnosed with ADHD. Through family, twin and adoption studies, ADHD has now been established as a complex disorder with both genetic and environmental components [Lopez, 1965; Morrison and Stewart, 1971, 1973; Cantwell, 1972, 1975; Welner et al., 1977; Faraone et al., 1994; Doyle et al., 2001]. With a heritability estimate of 0.76 [Faraone et al., 2005], evidence that the etiology of ADHD is partially due to genes is compelling.
The heterogeneity inherent in the definition of ADHD suggests there may be genes that influence specific characteristics of ADHD. This concept has been supported by evidence that there may be genes associated with specific ADHD subtypes [Neuman et al., 1999; Rasmussen et al., 2004]. Research has found that specific candidate genes are associated with specific subtypes of ADHD. For example, the common 148-bp allele of a microsatellite marker located 18.5 kb from the D5 dopamine receptor gene (DRD5) has been associated with the inattentive and combined subtypes, but not the hyperactive-impulsive subtype [Lowe et al., 2004]. It should be noted, however, that with the low prevalence of the hyperactive-impulsive subtype in this sample, there was likely limited power to detect this association. In addition, several twin studies [Todd et al., 2001; Rasmussen et al., 2004] have demonstrated that the transmission of inattentive and hyperactive-impulsive symptoms is distinct. Findings such as this suggest that studying ADHD subtypes represents one strategy for identifying genes associated with the disorder.
Another strategy for identifying genes associated with a heterogeneous disorder like ADHD is through phenotype refinement. Alternative phenotypic definitions, such as quantitative measurements that describe specific characteristics of a disorder provide one option to better handle the heterogeneity of ADHD. As Todd [2000] has suggested, a complete understanding of the genetics of ADHD may require better characterization of heritable quantitative phenotypes underlying ADHD and a better understanding of its genetic heterogeneity. Alternative definitions of ADHD, through the use of statistically derived phenotypes, may help by providing stronger “signals” in molecular genetics research.
Several genes within the dopaminergic system have been implicated in ADHD primarily because of their known physiological involvement in the functioning of stimulant medications. The dopamine transporter gene (DAT), located at 5p15.3, was first considered a good candidate gene for ADHD because of its biological function; the therapeutic effects of stimulant medications, often used to treat ADHD, result from blocking the dopamine transporter [Spencer et al., 2000]. DRD5, located at 4p16.1, is also considered a good candidate gene for ADHD. A pooled analysis of 14 studies of the 148 bp allele of a microsatellite marker located 18.5 kb from DRD5 identified an association with inattentive and combined ADHD subtypes [Lowe et al., 2004]. Several lines of biologic evidence implicate synaptosomal-associated protein 25 (SNAP-25) mutations, located at 20p12-p11.2, in ADHD etiology. Animal studies of the coloboma mouse mutant suggest that it models the hyperactivity observed in ADHD [Hess et al., 1992]. Furthermore, studies have shown that the deletion of the SNAP-25 gene in this mutant is the primary cause of the hyperactivity [Hess et al., 1996], supporting the possibility that variants in SNAP-25 may be involved in the etiology of ADHD. Several association studies have examined different mutations of SNAP-25 with positive findings [Faraone et al., 2005].
One of the best-studied genes associated with ADHD is the D4 dopamine receptor gene (DRD4), which is located at 11p15.5. DRD4 has been frequently implicated as a candidate gene for ADHD based on its function; the distribution of DRD4 mRNA in the brain suggests that it plays a role in clinical characteristics of ADHD, specifically cognitive and emotional functioning [Paterson et al., 1999]. Meta-analytic findings using 21 studies show that the 7-repeat allele in DRD4 is significantly associated with ADHD [Faraone et al., 2001]. Additional studies have also used quantitative phenotypes to study the relationship between DRD4 and ADHD. Significant associations between the DRD4 7-repeat allele and both variables from neuropsychological tests and attention data have been found [Manor et al., 2002; Langley et al., 2004]. Use of other phenotypes, such as longitudinal measures of hyperactivity and ADHD symptom counts data have not found evidence of significant relationships with DRD4 [Fisher et al., 2002; Mill et al., 2002].
While the serotonergic system has been less frequently implicated in ADHD, several lines of evidence suggest a possible role in the neurobiology of the disorder. Puumala and Sirvio [1998] studied the variability in attention and impulsive behavior through neurochemical assays of rat brains and found that frontal cortex dopamine and serotonin were both important in the regulation of attention and response control. The 5HT1B serotonin receptor (HTR1B) gene, located at 6q13, represents one gene in the serotonergic system that is a viable candidate gene for ADHD as a pooled analysis of five family-based association studies reported an association between the 861G variant of HTR1B and ADHD [Hawi et al., 2002].
As reviewed by Faraone et al. [2005] both biologic plausibility and positive meta-analytic findings suggest that DAT, DRD4, DRD5, SNAP-25, and HTR1B are strong candidate genes for ADHD and therefore these genes we selected for further study. However, more remains to be discovered about their potential role in the etiology of the disorder. Although prior research has used complex phenotypes in association analyses [Manor et al., 2002; Mill et al., 2002; Langley et al., 2004], these studies have not developed maximally heritable phenotypes that have high power to detect an association with markers in or near these candidate genes. In this article we use ADHD symptom dimensions to generate such phenotypes for each single nucleotide polymorphism (SNP) throughout the ADHD candidate genes. By generating these phenotypes, we hope to further clarify the role that these genes may have in the etiology of ADHD.
METHODS
Study Population
Two hundred twenty-nine ADHD families were recruited through several ongoing research studies being conducted at Massachusetts General Hospital pediatric psychopharmacology clinic (MGHPPC): (1) 90 from the longitudinal case-control family studies of boys and girls; (2) 83 from an affected sibling pair linkage study of ADHD; (3) 37 from a family study of bipolar disorder; (4) 17 from a family study of ADHD adults and; (5) 2 from a study of ADHD and substance abuse. Because these studies were conducted by the same research group, the ascertainment criterion for ADHD did not differ among studies, for example, children enrolled as bipolar probands for the family study of bipolar disorder would have qualified for enrollment in the ADHD studies if they also met criteria for ADHD. For the longitudinal case-control family studies of boys and girls, probands were recruited from either MGHPPC or from HMOs in the Boston area. Ascertainment of the probands and their relatives was based on DSM-III-R criteria as subjects were recruited before the publication of DSM-IV. Individuals of 6–18 years of age were eligible to participate in this study. Potential subjects were excluded if they were adopted, had major sensorimotor handicaps, psychosis, autism, inadequate command of the English language, an IQ less than 80, or their nuclear family was not able to participate in the study. All of the ADHD probands met DSM-III-R diagnostic criteria for ADHD at the time of the clinical referral and had active ADHD symptoms at the time of recruitment. Recruitment, inclusion, and exclusion criteria for the other studies listed above were the same as the longitudinal study for ADHD boys and girls with the following exceptions: (1) ADHD cases were obtained from the MGHPPC, the child psychiatry clinic at Children's Hospital in Boston, or by referrals from individual child psychiatrists throughout the community; (2) ascertainment was based on DSM-IV diagnoses; (3) the pediatric bipolar studies ascertained cases for bipolar disorder and did not screen out cases with psychosis. Individuals 18 years of age or older provided written informed consent, mothers provided written informed consent for minor children and children provided written assent to participate in this study.
ADHD Diagnostic Assessment
Psychiatric information was collected from children using the K-SADS-E (Epidemiologic Version) for either DSM-III-R or DSM-IV. The K-SADS-E is a widely used semi-structured psychiatric diagnostic interview with established psychometric properties [Orvaschel, 1994]. The interview inquires about the child's lifetime history of psychopathology and provides a standardized method of obtaining and recording symptoms necessary for the assessment of several disorders, including ADHD. The information collected for ADHD included nine inattentive and nine hyperactive-impulsive symptoms. Each symptom was recorded as present (one) or absent (zero); in the few cases where there was ambiguity the individual was coded as half.
For all children, psychiatric data were collected from the mother. In addition, children 12 and older were evaluated directly. For individual symptoms, data were combined such that if either the mother or youth endorsed the symptom, it was counted as present. Youth under 12 were not interviewed directly because there is debate as to the extent to which a child's self-perceptions, memories, feelings and reported behavior can be reliably assessed through self-report. Although limited, studies on the use of interview techniques among young children show that their replies are unreliable. For example, Breton et al. [1995] showed that children aged 9–11 understood only about 40% of the questions from the Diagnostic Interview Schedule for Children (Version 2.5). Consistent with this, Edelbrock et al. [1985] and Schwab-Stone et al. [1994] reported poor reliability of reports of psychopathology from children younger than 12. In contrast, Faraone et al. [1995] have shown maternal reports of psychopathology to reach high levels of reliability, even over a 1-year period. Final diagnostic assignment for ADHD was made after blind review of all available information by a diagnostic committee chaired by Dr. Biederman and composed of three board-certified child and adolescent psychiatrists and licensed clinical psychologists.
Genotyping Methods
One hundred forty-three SNPs were selected across five ADHD candidate genes: 35 SNPs for DAT, 13 SNPs for DRD4, 13 SNPs for DRD5, 63 SNPs for SNAP-25, and 21 SNPs for HTR1B. These SNPs were selected to cover each candidate gene and their flanking regions to allow for better characterization of the haplotype block structure. To evaluate SNP assay quality and characterize the linkage disequilibrium (LD) relationships we screened the SNPs in 12 multigenerational CEPH pedigrees. SNPs were selected for testing in the ADHD family sample if they met the pre-specified quality control metrics. Twelve multigenerational CEPH families and our SNP data were used to generate haplotype blocks of LD. The EM algorithm and the haplotype block criteria of Gabriel et al. [2002] were used to determine the LD structure, as implemented in the program Haploview [Barrett et al. 2005]. Genotyping of the SNPs was done by MALDI-TOF mass spectrometry [Buetow et al., 2001].
Family-Based Association Test-Principal Components (FBAT-PC)
Family-based association tests are those studies that use genetic data from family members to evaluate the possible association of a disease phenotype and a gene allele. FBAT-PC is an approach designed to maximize the genetic information when multiple phenotypes are tested [Lange et al., 2004b]; it has been implemented in the PBAT computer program [Lange et al., 2004a]. The FBAT-PC methodology has been extremely successful in identifying SNPs associated with complex diseases in genomewide association studies. Recently, a 100K scan of reported an obesity gene that met genomewide significance using this methodology with replication in several independent samples across various ages and ethnicities [Herbert et al., 2006].
When multiple phenotypes are used, FBAT-PC uses principal components analysis to construct an overall phenotype that amplifies the trait heritability by aggregating the genetic components of all measurements into a single univariate phenotype with maximal heritability. This univariate trait with maximal heritability is constructed as follows. First, the conditional mean model proposed by Lange et al. [2003] is used to generate genetic effect sizes for each phenotype. The effect size estimates are used along with information on the mode of inheritance and allele frequencies to estimate a matrix of genetic variances at that SNP. Second, the phenotypic variance matrix is calculated using the original set of phenotypes as well as any other desired covariates. Once the genetic and phenotypic variance matrices are generated, generalized principal components are used to identify the set of weights that maximize the heritability at the given SNP; heritability is calculated as the weighted combination of genetic variances divided by the weighted combination of the total variance (the sum of the genetic and phenotypic matrices). We apply this methodology to nine inattentive and nine hyperactive-impulsive symptoms for ADHD. The inattentive symptoms include: (1) inability to pay attention to details; (2) difficulty with sustained attention in tasks or play activities; (3) listening problems; (4) difficulty following instructions; (5) problems organizing tasks and activities; (6) avoidance or dislike of tasks that require mental effort; (7) tendency to lose things like toys, notebooks, or homework; (8) distractibility; and (9) forgetfulness in daily activities. Hyperactive-impulsivity symptoms include: (1) fidgeting or squirming; (2) difficulty remaining seated; (3) restlessness; (4) difficulty playing quietly; (5) always seeming to be “on the go;” (6) excessive talking; (7) blurting out answers before hearing the full question; (8) difficulty waiting for a turn or in line; and (9) problems with interrupting or intruding. Using only one of these ADHD symptoms does not provide as much information as using a phenotype that is generated from many of the symptoms combined using FBAT-PC.
Once the univariate phenotype is generated at each SNP, FBAT-PC employs a screening procedure that selects the SNPs to be tested using a univariate quantitative FBAT statistic. Such a statistic is called the FBAT-PC statistic. The screening procedure works as follows: (1) for each SNP, power to detect association with the generated univariate phenotype is calculated; (2) a group of SNPs with their associated phenotypes are selected based on the power to detect a genetic association and; (3) the FBAT-PC statistic is calculated on the SNPs and their associated phenotypes. In this analysis, the additive genetic model was used in the screening procedure and a minimum of 20 informative families were required for any given SNP to be screened. The five SNP/genetic model combinations with the greatest power were retained and the FBAT-PC statistic was calculated. These five SNPs were subsequently adjusted using the false discovery rate [Benjamini and Hochberg, 1995]. We adjusted the analysis for other psychiatric comorbidity using three approaches. In one approach, we included a count of the number of comorbid disorders that each individual had. The comorbid disorders that were accounted for in this count included the following: agoraphobia, alcoholism, anorexia, major depression, bipolar disorder, bulimia, conduct disorder, dysthymia, generalized anxiety disorder, obsessive-compulsive disorder, oppositional defiant disorder, substance abuse/dependence. In the second approach, we generated an indicator variable that was positive if the individual had at least one of the comorbid disorders. In the final approach, we included binary indicators for all of these comorbid disorders as covariates in the analysis simultaneously.
The contribution of each ADHD symptom to the final univariate phenotype was determined by first looking at the correlation of each ADHD symptom with the phenotype that was generated from the FBAT-PC program. We then ranked these correlations from the highest to the lowest to determine what symptoms were most strongly correlated with the generated phenotype. If the ranking of the correlations revealed that a clinically meaningful subgroup of ADHD symptoms, FBAT-PC was rerun with the selected group of ADHD symptoms and the P-value from the FBAT-PC statistic was directly evaluated at the SNPs previously found to be significant (i.e., no screening procedure was used).
RESULTS
Descriptive Statistics
Of the 229 available families, 228 had sufficient information to be used in this analysis. Descriptive information on these families is listed in Table I. The distribution of responses to the inattentive and hyperactive-impulsive symptoms among children is listed in Table II.
| Number of people | 758 |
| Number of families | 228 |
| Number of affected siblings within a family | |
| 1 | 129 |
| 2 | 84 |
| 3 | 12 |
| 4 | 3 |
| ADHD subtypes (percentage) among children | |
| Inattentive | 108 (32.7) |
| Hyperactive-impulsive | 20 (6.1) |
| Combined | 202 (61.2) |
| Missing | 15 |
| Gender distribution (percentage) among children | |
| Male | 210 (60.9) |
| Female | 135 (30.1) |
| Inattentive symptoms | Absent | Unclear | Present |
|---|---|---|---|
| Inability to pay attention to details | 17.0 | 1.3 | 81.7 |
| Difficulty with sustained attention in tasks or play activities | 1.7 | 0.3 | 98.0 |
| Apparent listening problems | 8.1 | 1.2 | 90.7 |
| Difficulty following instructions | 8.1 | 1.5 | 90.4 |
| Problems organizing tasks and activities | 10.6 | 1.0 | 88.4 |
| Avoidance or dislike of tasks that require mental effort | 15.1 | 0.6 | 84.2 |
| Tendency to lose things like toys, notebooks, or homework | 20.0 | 0.9 | 79.1 |
| Distractibility | 1.7 | 0.6 | 97.7 |
| Forgetfulness in daily activities | 21.9 | 1.0 | 77.2 |
| Hyperactive-impulsive symptoms | Present | Unclear | Absent |
|---|---|---|---|
| Fidgeting or squirming | 13.0 | 1.7 | 85.2 |
| Difficulty remaining seated | 21.7 | 0.9 | 77.4 |
| Excessive running or climbing | 26.8 | 1.3 | 71.9 |
| Difficulty playing quietly | 46.7 | 2.3 | 51.0 |
| Always seeming to be “on the go” | 39.8 | 3.1 | 57.1 |
| Excessive talking | 36.2 | 2.9 | 60.9 |
| Blurting out answers before hearing the full question | 21.5 | 2.9 | 75.7 |
| Difficulty waiting for a turn or in line | 27.8 | 2.6 | 69.6 |
| Problems with interrupting or intruding | 14.5 | 2.0 | 83.5 |
FBAT-PC Analysis
Table III shows the five SNPs selected through the screening procedure listed from highest to lowest power within each candidate gene. Also presented are the number of informative families at each SNP and the FBAT-PC P-value. After adjusting for multiple comparisons, adjacent SNPs hCV26775267 and hCV26775266 in the promoter region of DRDR4 remained significant (FDR corrected P-value hCV26775267 = 0.012, FDR corrected P-value for hCV26775266 = 0.012). The over-transmitted allele for hCV26775267 and hCV26775266 was C and T, respectively. When additional psychiatric comorbidities were adjusted for in the analysis, the significance of the findings did not change. The significant SNPs hCV26775267 and hCV26775266 were found to be strong LD in this sample (D′ = 1.0 [95% CI = 0.99, 1.00]; rounded r2 = 1.0). These SNPs are located within 0.4 kb of the transcription start of DRD4 (http://snpper.chip.org/bio) and were in modest LD with the exon 3, 7-repeat variant (D′ = 0.93 [95% CI = 0.78, 0.98]; r2 = 0.14). The function of both SNPs is unknown.
| Candidate gene | Marker | Number of informative families | Unadjusted FBAT-PC P-value |
|---|---|---|---|
| DRD4 | rs7932167 | 113 | 0.592 |
| DRD4 | rs3758653 | 98 | 0.916 |
| DRD4 | hCV26775267 | 146 | 0.005a |
| DRD4 | hCV26775266 | 144 | 0.002a |
| DRD4 | rs1124816 | 97 | 0.123 |
| DRD5 | hCV12062485 | 111 | 0.560 |
| DRD5 | rs1519096 | 114 | 0.209 |
| DRD5 | hCV12062484 | 111 | 0.588 |
| DRD5 | rs7690455 | 140 | 0.108 |
| DRD5 | rs3762933 | 157 | 0.211 |
| HTR1B | rs1160077 | 146 | 0.690 |
| HTR1B | rs6296 | 128 | 0.480 |
| HTR1B | rs1145830 | 116 | 0.043 |
| HTR1B | rs1145827 | 83 | 0.561 |
| HTR1B | rs6298 | 126 | 0.637 |
| DAT | rs2245660 | 25 | 0.683 |
| DAT | rs6347 | 121 | 0.539 |
| DAT | rs2963257 | 139 | 0.849 |
| DAT | hCV2854696 | 60 | 0.997 |
| DAT | rs4975544 | 123 | 0.432 |
| SNAP-25 | rs362585 | 33 | 0.610 |
| SNAP-25 | rs6074121 | 134 | 0.062 |
| SNAP-25 | rs362552 | 135 | 0.070 |
| SNAP-25 | rs362988 | 139 | 0.330 |
| SNAP-25 | rs2423486 | 100 | 0.395 |
- a Indicates that the SNP is significant at the α = 0.05 level after adjusting for multiple comparisons using the FDR correction.
The significant SNPs hCV26775267 and hCV26775266 were on the same haplotype block as determined by both this sample and the CEPH families. Therefore, an FBAT-PC analysis was performed on the four SNP haplotype block in which the two significant SNPs reside. One of the four haplotypes tested in haplotype block rs936464:rs936465:hCV26775267:hCV26775266 was significantly associated with the univariate phenotype (P-value for haplotype A:G:C:T = 0.008, P-value for haplotype G:C:T:C = 0.265, P-value for haplotype A:G:T:C = 0.446; P-value for haplotype G:C:C:T = 0.264). The significant P-value was associated with an over-transmission of haplotype A:G:C:T.
Symptom Correlations
The correlations between each ADHD symptom and the overall phenotypes for hCV26775267 are shown in Figure 1. At hCV26775267, 9 of the 11 highest correlations are with inattentive symptoms. Only two hyperactive-impulsive symptoms, restlessness and excessive fidgeting/squirming, are among the top-ranked correlations. The correlations for hCV26775266 are similar, with all nine inattentive symptoms being ranked among the 12 greatest correlations. Three hyperactive-impulsive symptoms also had high correlations: restlessness, excessive fidgeting/squirming, and excessive talking. It is not surprising that the correlations are so similar at the two SNPs, as they are in very strong LD with each other. The apparent strong influence of inattentive symptoms on the overall univariate phenotype was evaluated by rerunning the FBAT-PC analysis for inattentive and hyperactive-impulsive symptoms separately. The P-value for the FBAT-PC statistic was evaluated directly at hCV26775267 and hCV26775266 (i.e., no screening procedure was used). When inattentive symptoms were used to generate a univariate phenotype and the FBAT-PC statistic was evaluated at hCV26775267 and hCV26775266, these SNPs remained significant (unadjusted P-value for SNP hCV26775267 = 0.034; unadjusted P-value for SNP hCV26775266 = 0.017) with the same over-transmitted alleles. When hyperactive-impulsive symptoms were used to generate a univariate phenotype and the FBAT-PC statistic was evaluated at hCV26775267 and hCV26775266, neither SNP remained significant.
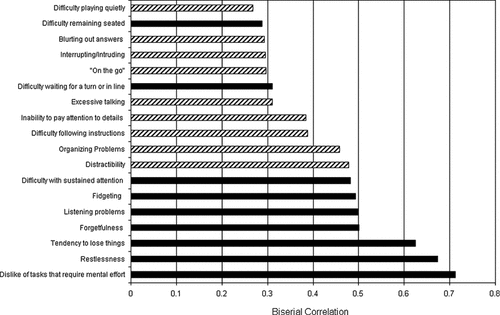
Correlations of ADHD symptoms with phenotype.
DISCUSSION
Through the use of a novel analytic method, we were able to identify an association between two SNPs in the DRD4 promoter region and maximally heritable quantitative phenotypes based on nine inattentive and nine hyperactive-impulsive ADHD symptoms. The high correlations between the inattentive ADHD symptoms and the quantitative phenotype suggest that the inattentive symptoms are more influential in generating the overall phenotype used the analysis. Furthermore, it appears as if the inattentive symptoms are also primarily responsible for the observed associations, as repeating the FBAT-PC analysis with only inattentive symptoms continued to result in a significant association while this is not true when only hyperactive-impulsive symptoms were used in the analysis. The strong LD that exists between hCV26775267 and hCV26775266 explains the consistent findings that are observed between these two SNPs.
Previous studies have reported an association between ADHD and the 7-repeat variant in exon 3 of DRD4 [Faraone et al., 2001]. Interestingly, hCV26775267 and hCV26775266 are in LD with rs7124601 (D′ = 0.98; [95% CI = 0.94, 1.0]; r2 = 0.67), which is located in the same exon as the 7-repeat allele, suggesting that our finding may also be in LD with the 7-repeat allele. Therefore, previous association findings at this microsatellite may be identifying the same susceptibility locus as the SNPs are identifying here.
Our finding suggests that either hCV26775267 and hCV26775266 or a functional variant in LD with these SNPs may be important in influencing the inattentive symptoms of ADHD. Seeing that the SNPs are in the promoter region, new efforts should include the promoter to determine if this region is influencing inattentive symptoms. These results do not explain exactly how this region is acting to influence inattention. After independent replication of these findings, future research should focus on identifying the functional variant in this region. Functional studies of DRD4 in animal models may be useful to determine if DRD4 influences inattention, as such studies have successfully identified that DRD4 plays a role in hyperactivity [Avale et al., 2004].
Although no studies report our specific finding, prior research has found some associations between inattention and the DRD4 gene in ADHD families. McCracken et al. [2000] found an association between the 240 bp repeat promoter polymorphism in DRD4 and individuals with the inattentive subtype. Because our finding is also in the promoter region, it is possible that what we are observing is related to this finding, although the casual variant remains to be determined. This analysis is different from ours in that we did not limit our sample to those with the inattentive subtype. Rather, we looked at inattentive symptomatology among all individuals with ADHD. Therefore, our findings extend to a broader group of individuals than previously reported. Rowe et al. [2001] retrospectively evaluated the level of inattention that fathers of ADHD and control children had in adolescence and found that fathers with the 7-repeat allele in DRD4 had increased symptoms of inattention compared with control fathers. Our study adds to this literature by showing that an association in the promoter region of DRD4 with a refined, heritable phenotype is strongly correlated with inattentive symptoms.
Notably four of the five candidate genes that we were investigating had no significant findings. This could be explained in several ways. First, this could be due to the sample size. Although the sample is not small, genes with small effects require very large sample sizes in order to detect the effect so it is possible that we could still miss genetic effects. Second, this could suggest that, although these genes are associated with ADHD, they do not regulate the degree or nature of symptom expression. Finally, it is always possible that some of these genes are not actually associated with ADHD, although we consider that less likely given that each gene has been studied by several independent laboratories and meta-analyses of combined data indicate there is an association [Faraone et al., 2005].
There are several limitations of this study. By selecting the five SNPs with greatest power at each candidate gene, the FBAT-PC screening procedure may have missed some significant results that had acceptable power but were not among the five SNPs with greatest power. However, without the screening procedure the FDR adjustment would be severe and therefore we would likely miss the significant associations with other testing strategies as well. The ADHD families used in this analysis were ascertained through different studies. Because some of these studies had ascertainment criteria other than ADHD (e.g., selecting through bipolar probands, requiring two or more ADHD siblings) our work may not generalizable to samples recruited only through a single ADHD proband. Although families were recruited using slightly different diagnostic systems (DSM-III-R or DSM-IV), the collection of data on inattentive and hyperactive-impulsive symptoms was uniform throughout the sample and the correspondence of ADHD diagnoses between these systems is high [Biederman et al., 1997]. Finally, hyperactive-impulsive symptoms tend to be more variable and less reliable than inattentive symptoms, which would decrease the estimated heritability for the former symptoms. Such differences in reliability would result in greater power to detect genetic associations for inattentive compared with hyperactive-impulsive symptoms when in the magnitude of association is similar for both groups.
Despite these limitations, this study suggests that two SNPs in the DRD4 promoter region, hCV26775267 and hCV26775266, appear to be affecting inattention in ADHD. This finding is consistent with ADHD being a genetically heterogeneous disease. Future studies should try to identify a causal variant, examine the relationship between this signal and the 7-repeat allele, and to see if these particular SNPs are functional in some way.
Acknowledgements
This work was sponsored by the National Institute of Mental Health through the Psychiatric Epidemiology and Biostatistics training grant (T32-MH017119) as well as three grants from the National Institute of Health: R01HD37694, R01HD37999, and R01MH66877. We thank Dr. Pamela Sklar for her useful comments on this manuscript.