Decomposing the autism phenotype into familial dimensions†
Please cite this article as follows: Szatmari P, Mérette C, Emond C, Zwaigenbaum L, Jones MB, Maziade M, Roy M-A, Palmour R. 2007. Decomposing the Autism Phenotype Into Familial Dimensions. Am J Med Genet Part B 147B:3–9.
Abstract
The objective of this article is to decompose the level of functioning phenotype in autism to see if it can be conceptualized as two simpler, but still familial, dimensional phenotypes of language and non-verbal IQ. We assembled 80 sibpairs with either autism, Asperger syndrome or atypical autism. To see whether the familial correlation on language scores was accounted for by the familial correlation on non-verbal IQ, residual language scores were calculated for each member of the sibpair based on a multiple regression equation using their IQ score as an explanatory or independent variable and controlling for the age and gender of the affected individual. These residual scores were then used to calculate intraclass correlations between affected sibs. This process was repeated using IQ as the dependent variable and language as a covariate. Within affected individuals there was a strong relation between non-verbal IQ (as measured by the Leiter performance scale) and language (as measured by the Vineland Communication Scale). In addition, there was familial correlation between sibs on both measures. Evidence of familial aggregation on both non-verbal IQ and language remained even after partialling out the effect of the covariates by regression analysis and by generalized estimating equation. These findings suggest that non-verbal IQ and language in PDD may arise from independent genetic mechanisms. The implications of this finding for linkage analysis and for identifying genetically informative phenotypes are discussed. © 2007 Wiley-Liss, Inc.
INTRODUCTION
Autism is a disorder that shows considerable variation in clinical expression. Some children can have many symptoms, others just a few, and the number and type of symptoms may change with development [Piven et al., 1996; Starr et al., 2003]. There are also different “pervasive developmental disorders” (PDDs) that share Wing's triad of social impairments in reciprocal interaction, in verbal and non-verbal communication and in a preference for repetitive activities rather than social forms of play [Wing, 1996]. But many in the field see the distinctions between these “subtypes” as largely artificial and not all that useful in efforts to find autism-related genes. Many prefer to think of a “spectrum” of disorders [Wing, 1996] that differ on a continuum of severity. The factors that account for this variation, however, remain poorly understood.
Rapid advances have been made recently in understanding the etiology of autism, notably the fact that genetic mechanisms account for a large part of etiology [Cook, 2001]. Older studies reported that the risk to siblings is between 50 and 100 times the population prevalence and the twin studies provided estimates of heritability of greater than 90% [Szatmari et al., 1998]. While these figures are widely quoted [Folstein and Rosen-Sheidley, 2001] they may need to be revised downward given the evidence that the disorder is more common in the general population than previously thought [Chakrabarti and Fombonne, 2001]. While it should be relatively easy to identify susceptibility genes given these estimates, there are now several genome scans published, none of which have been able to identify regions of genome-wide significance [for reviews see Wassink et al., 2004; Bacchelli and Maestrini 2006; Coon, 2006]. The most recent scan with the largest sample size has perhaps the most promising results [Szatmari et al., 2007a] but still the most significant result is part of sub-group analyses.
There are of course many possible reasons why this situation may exist [Szatmari et al., 1998]. Foremost among these is the possibility that the phenotypes used in these genome scans may not always be the most informative for genetic purposes [see Baron, 1995; Tsuang and Faraone, 2000 for similar arguments]. For the most part, the phenotype used in current genetic studies consists of a diagnosis of autism (broad or narrow) as conceptualized in DSM-IV and as operationalized by the Autism Diagnostic Interview (ADI). This may be helpful in some circumstances but it still includes a very wide range of symptoms and a level of functioning (LOF) that can range from profound developmental delay to functioning well above average. It is certainly possible, given the enormous range of clinical variation seen in autism, that cases at the ends of the spectrum might arise from separate genetic mechanisms. It is also possible that different components of the phenotype are associated with different genetic mechanisms between families. Affected individuals from the same family may be concordant for some aspect of the phenotype but not others, possibly reflecting intrafamily locus heterogeneity. This would be especially true if the genes causing the disorder were relatively common.
Thus, searching for more genetically informative phenotypes in autism is an essential prerequisite to the detection of susceptibility genes [Szatmari et al., 2007b]. Attempts to subtype autism have a long and checkered history [Beglinger and Smith, 2001]. The problem is that the process for defining subtypes has not been guided by genetic epidemiologic methods [Tsuang, 2001]. If the goal is to identify susceptibility genes, then the subtypes, or component phenotypes, must be defined with that purpose in mind. A genetically informative phenotype is one where the risk of that phenotype at a specific genetic locus is very high [Szatmari et al., 2006]. As a result, the mode of transmission of that specific phenotype within a pedigree may be simpler, and more Mendelian, than other definitions of the phenotype. Some attempts to use more informative phenotypes in autism such as those based on quantitative traits have indeed been more successful in garnering marginally higher linkage signals than traditional categorical-diagnostic approaches, a strategy that supports the potential utility of this approach [Alarcon et al., 2002, 2005; Buxbaum et al., 2004; Chen et al., 2006]. The key issue in this context, though, is which dimension to use since there are so many from which to choose.
Thus, the search for genetically informative phenotypes in autism may be complicated by the possibility that the disorder is a multivariate phenotype; that is, made up of two or more dimensions that may or may not be correlated with each other. Ronald et al. [2006] have recently argued, using twin data from the general population, that autistic symptoms are composed of two independent dimensions; social reciprocity and repetitive behaviors. In a similar vein, we have recently shown using factor analysis on a sample of affected subjects that measures of LOF and autistic symptoms represent two domains where the observable variables are psychometrically correlated but the underlying factors are essentially orthogonal [Szatmari et al., 2002]. LOF refers in this context to the actual achievement of important developmental milestones in everyday functioning. It is usually assessed using IQ and measures of socialization and language from the Vineland Adaptive Behavior Scales [VABS; Volkmar et al., 1993; Carter et al., 1998]. We have begun to use LOF to identify more informative phenotypes in PDDs. For example, we have shown that both non-verbal IQ and language skills are the most familial characteristics among sibships with multiple cases of PDD [MacLean et al., 1999]. Significant familial aggregation indicates that the variance between sibships on these dimensions is greater than the variance within sibships. Insistence on sameness also shows familial aggregation in our, and in other, data sets [Silverman et al., 2002; Szatmari et al., 2006], however, the magnitude of the estimate appears lower suggesting that language and non-verbal IQ might be more useful traits to use in linkage analysis. The problem is that, intuitively, non-verbal IQ and communication skills are likely to be highly correlated within a population. Thus, it is not clear that the familial aggregation observed on IQ is independent of the familial correlation on communication. If it is the genetic architecture underlying one dimension may be different from the architecture underlying the other, so combining them into one domain (such as LOF) may not be appropriate. This would also have important implications for linkage analysis in that it would suggest that genes linked to one dimension may not be linked to the other. Searching for linkage to these dimensions separately may be a more powerful strategy than searching for genes linked to autism as a whole.
SAMPLE
Multiple incidence or multiplex (MPX) families were recruited systematically from parent support groups and mental health and social service agencies across Canada that serve children with developmental disorders, [see MacLean et al., 1999 for complete description of recruiting]. We ascertained families with two or more siblings with any form of PDD (including autism, atypical autism, PDD (NOS) not otherwise specified, Asperger syndrome, and disintegrative disorder, but not Rett's syndrome). No restriction was placed on PDD subtype or on LOF. After a full description of the study was given to the families, written informed consent was obtained from the parents as well as from children over 18 years who were able to give informed consent. Seventy-six MPX families with 156 affected children were recruited (Table I). Of these 76 MPX families, 72 consisted of sibpairs and 4 families contained 3 affected children. For each of these latter families, only two independent pairs among the 3 possible sibpairs were selected to yield a total of 80 affected sibpairs in the first analysis reported below. The sibpairs reported in the Maclean et al.'s article [1999] are a subset of this sample. We identified the proband as the first child to be identified as affected.
| Probands | Siblings | |||
|---|---|---|---|---|
| N | Mean (SD) or percentage | N | Mean (SD) or percentage | |
| Number of males | 76 | 84% | 80 | 86% |
| Leiter IQ | 76 | 67 (28) | 80 | 65 (31) |
| VABS Comm | 70 | 64 (26) | 75 | 62 (20) |
| ADI-R domains; Social interaction | 76 | 24 (4) | 80 | 23 (5) |
| Verbal Comm | 39 | 18 (4) | 34 | 17 (5) |
| NV Comm | 37 | 13 (1) | 46 | 13 (2) |
| Repetitive B | 76 | 7 (2) | 80 | 6 (3) |
- Comm, communication; NV, non-verbal; B, Behavior.
The following additional inclusion criteria were applied if the proband received a diagnosis of PDD by DSM-IV criteria: (1) age greater than two years for the affected children, (2) English being the language most often spoken in the home, and (3) no neurological disease or known chromosomal disorder. Medical records were checked to ensure that all probands and affected siblings had undergone medical evaluations including metabolic screens, EEGs, DNA testing for the fragile-X syndrome and routine karyotyping to rule out known neurologic and chromosomal disorder. The mean age of the probands was 112 months (SD = 75; range 28–482); and of the sibs, the mean was 90 (SD = 71; range 28–359).
PROCEDURE
Diagnosis and Assessments on Probands and Affected Siblings
For all but 13 families, the proband was the oldest of the siblings with PDD. Subsequent to the identification of a potential “proband,” clinical assessments were carried out on all children in the sibship to distinguish affected and unaffected children. Parts of the Family History Interview for Developmental Disorders of Cognition and Social Functioning [Folstein and Rutter, 1991] were used to screen all children. If any PDD behaviors were observed, the ADI and later the ADI-R [Lord et al., 1994] was used to collect information to make a more definite diagnosis. The Autism Diagnostic Observation Scale [ADOS; Lord et al., 1989] and its newer version [Lord et al., 2000] were also used to assess the social, language, and play skills of the child based on direct observation. Finally, clinical records were obtained but information on previous diagnoses was deleted. An independent blind diagnosis was then made, according to DSM-IV criteria, [American Psychiatric Association, 1994] using the ADI-R, the ADOS and all available clinical information by three clinicians (none of whom had seen the subject) with an average of 20 years experience in the diagnosis of PDD children. If there was any disagreement, the case was discussed and a best-estimate consensus diagnosis was reached. A sample of non-PDD cases were also diagnosed by the panel so that each multiplex case was diagnosed blind to the status of that individual's siblings and to avoid any expectation bias (see Mahoney et al. [1998] for a full description of diagnostic procedures, reliability and accuracy estimates). Of the 156 cases, 115 were given a diagnosis by best estimate of autism, 18 had atypical autism, and 23 were given a diagnosis of AS. The mean and standard deviation of the age, IQ, and ADI scores of the sample are given in Table I and indicate that this is a representative sample of children with PDDs with a typically wide distribution of scores. Seventy-three subjects were verbal and 83 were non-verbal.
The Leiter IQ scale was also completed on affected children and the VABS was completed either in face-to-face interviews or over the phone for those who lived at a distance. In all cases, the primary care giver who completed the ADI-R was the mother. The same interviewer conducted the interview for all siblings within a family but the ADI-R and the VABS were done by separate interviewers blind to each other's assessments.
INSTRUMENTS AND MEASURES
Autism Diagnostic Interview-Revised (ADI-R)
This is an investigator-based interview administered by a trained interviewer to the primary care giver(s) of the child [Lord et al., 1994]. It is designed to obtain detailed descriptions of behaviors necessary for the diagnosis of PDD, especially autism [Lord et al., 1994]. The interview focuses on the key diagnostic features described in the International Classification of Diseases-10th edition (ICD-10) and DSM-IV. The questions are designed to distinguish qualitative impairments from developmental delays by assessing behaviors at an appropriate age, identifying behaviors that would be considered deviant at any age and examining current and most abnormal behaviors for those strongly influenced by maturational age.
Autism Diagnostic Observation Schedule (ADOS)
The Autism Diagnostic Observation Schedule (ADOS) is a semi-structured assessment of communication, social interaction and play or imaginative use of materials, for individuals suspected of having autism or other PDDs. The ADOS consists of four modules, each of which is appropriate for children and adults of differing language levels, ranging from non-verbal to verbally fluent [Lord et al., 2000].
Leiter Performance Scales
This is the standard measure of non-verbal problem solving and learning ability [Levine, 1986]. It is especially appropriate to this population of PDD children because it does not require verbal instructions or responses for administration and correlates highly with WISC-R IQ. It is commonly used with PDD and other language impaired children. Both the old and the newer versions of the Lieter were used. All scores from the older version were adjusted to be consistent with the new version.
The Vineland Adaptive Behavior Scales
The VABS is a semistructured interview administered to a parent. This scale is designed to assess adaptive behavior in the domains of socialization, language and communication, motor and daily living skills [Sparrow et al., 1984]. The communication and socialization scores, and their relation to parameters such as IQ, are seen as very sensitive measures of impairment in children with PDD [Volkmar et al., 1993; Carter et al., 1998]. We report here on the communication scale (VABSCOM) alone which is standardized to a mean of 100 and a standard deviation of 15, with high scores indicating a higher language skills. It is composed of three sub-domains; receptive, expressive, and written language, but we used only the total score to ensure greater reliability.
ANALYSIS
To evaluate the genetic structure of language skills (VABSCOM) and non-verbal IQ we used two approaches first suggested by Raskind et al. [2000]. In our first approach, we used regression analyses on the independent sibpairs to estimate two models. In one model, IQ was included as a covariate (as well as age and gender) in the analysis where the subject's VABSCOM is treated as the dependent variable. Then, in the second model, an analysis was done where the role of the measures were reversed (dependent variable = IQ, covariate = VABSCOM, age and gender). In each of these analyses, the residual values, which are calculated as observed values minus predicted values, were extracted. The residual value is the variability due to the response variable that remains once the variability of the covariates is removed. Using the residual values from both models (Model 1: VABSCOMi = β0 + β1 IQi + β2 agei + β3 sexi + εi; Model 2: IQi = β4 + β5 VABSCOMi + β6 agei + β7 sexi + εi), we then estimated familial aggregation through intraclass correlation coefficients [Armitage and Berry 1994] with corresponding 95% confidence intervals [Rosner and Bernard, 2006]. Residual values were used to estimate correlation coefficients instead of the raw data because we wanted to know if the familial aggregation remained once the variability of the covariates was removed.
There are three possible outcomes (see Fig. 1); if including the covariate does not reduce or eliminate this familial aggregation pattern, then the covariate probably does not uniquely account for the familiality of the other measure (as in Fig. 1a). In Figure 1b the covariate would reduce or account for the variation in both directions suggesting a common genetic mechanism. However, if the familial aggregation pattern is reduced or eliminated in one direction but not in the reverse (see Fig. 1c), this would suggest that one of the two covariates has its own genetic influence in addition to a genetic factor shared with the other covariate. In the example shown in Figure 1c, IQ shares a genetic influence with VABSCOM, represented by Gene 1. Thus, when IQ is a covariate, it accounts for the familial aggregation of VABSCOM. However, when VABSCOM is the covariate, then IQ still displays familial aggregation due to its own genetic influence (Gene 2 in Fig. 1c). We also introduced age and gender as covariates in the regression analyses, since the observed familial aggregation may be due to an age or a gender resemblance in the sibpairs.
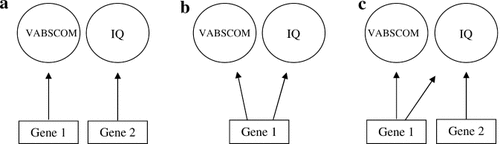
Theoretical illustration of three possible genetic systems underlying the expression of VABSCOM and IQ in autism: (a) VABSCOM and IQ have a completely different genetic influence, (b) VABSCOM and IQ share the same genetic factor, and (c) the IQ trait has its own genetic influence (Gene 2) in addition to a genetic factor shared with VABSCOM (Gene 1). The opposite is also possible but not illustrated.
As our second approach, a generalized estimating equation (GEE) was also used to estimate correlations between sibs [Hsu et al., 2002] while taking into account the correlation between language skills and non-verbal IQ. Two sets of estimating equations are computed. The first set estimates the marginal effects of covariates such as age and gender on each of IQ and VABS. The second set estimates the correlations between related pairs after adjusting for the covariates in the first set. These sets of equations are then solved simultaneously to obtain the estimates of intraclass correlation coefficients as well as regression coefficients of covariates [Hsu et al., 2002]. GEE can be conceptually written 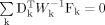 where the summation is over all K independent sibships (k = 1, …, K), Fk is the deviation of observed measure (VABSCOM in one model and IQ in the second model) and correlation functions from their expectations under the assumed mean and correlation regression models, Dk is a matrix of derivatives of the assumed marginal mean and correlation regression models with respect to unknown parameters, and finally, Wk is a matrix of weights [Raskind et al., 2000]. Here, the linear link function was used to model the marginal means and the marginal correlation. For this approach, we used the EE analysis program written and assembled by the Quantitative Genetic Epidemiology group at the Fred Hutchinson Cancer Research Center (see Q.G.E. Technical Report 126), and available at http://qge.fhcrc.org/software.php. This second approach offered the advantage of estimating within a single analysis, two ICCs while controlling for all covariates. It also offered the advantage of analyzing the original sample of 76 MPX families without having to select two different pairs in each of the 4 three-children families.
where the summation is over all K independent sibships (k = 1, …, K), Fk is the deviation of observed measure (VABSCOM in one model and IQ in the second model) and correlation functions from their expectations under the assumed mean and correlation regression models, Dk is a matrix of derivatives of the assumed marginal mean and correlation regression models with respect to unknown parameters, and finally, Wk is a matrix of weights [Raskind et al., 2000]. Here, the linear link function was used to model the marginal means and the marginal correlation. For this approach, we used the EE analysis program written and assembled by the Quantitative Genetic Epidemiology group at the Fred Hutchinson Cancer Research Center (see Q.G.E. Technical Report 126), and available at http://qge.fhcrc.org/software.php. This second approach offered the advantage of estimating within a single analysis, two ICCs while controlling for all covariates. It also offered the advantage of analyzing the original sample of 76 MPX families without having to select two different pairs in each of the 4 three-children families.
RESULTS
Our objective was to evaluate the structure of underlying shared and unique genetic contributions of communication skills and non-verbal IQ among the sample of 80 sibpairs affected by autism spectrum disorder. The two measures were highly correlated (r = 0.70; P-value < 0.0001) within affected subjects. The objective, however, was to determine if these two variables have a completely different genetic influence (Fig. 1a), if they share the same genetic factor (Fig. 1b) or if one of them has its own genetic influence (Fig. 1c).
Familial Aggregation of VABSCOM and IQ
The variables were highly correlated with each other supporting the need for taking the covariates into account. The covariates IQ, age and gender explained 50% of the variance of VABSCOM, while the covariates VABSCOM, age and gender explained 49% of the variance of IQ.
The intraclass correlation coefficient for the VABSCOM and IQ was calculated (correcting only for age and gender). There was positive evidence of familial aggregation on both these variables (i.e., VABSCOM: r1 = 0.25; IQ: r1 = 0.28, see Table II) but the 95% CI on these values was very wide. The intraclass correlation coefficients were then calculated using (instead of the scores only adjusted for age and gender) the residual values from the two regression models above. The ICCs remained surprisingly highly significant (VABSCOM: rIad = 0.36; IQ rIad = 0.39, see Table II) and in fact increased slightly.
| Outcome variables | Covariate | ICC | 95% CI | P-value |
|---|---|---|---|---|
| VABSCOM | Age, gender | 0.25 | 0.04–0.45 | 0.01 |
| IQ | Age, gender | 0.28 | 0.07–0.47 | 0.005 |
| VABSCOM | Age, gender, IQ | 0.36 | 0.16–0.54 | 0.0004 |
| IQ | Age, gender, VABSCOM | 0.39 | 0.19–0.56 | 0.0001 |
- VABSCOM, Vineland Communication Score.
The second approach used the GEE. The correlation coefficients which estimate familial aggregation of a variable accounting for the residual effects of the other variables was still moderately high and statistically significant (VABSCOM: rIad = 0.36; IQ rIad = 0.40, see Table III). Though the confidence intervals on these estimates were still rather wide.
| Outcome variable | Correlation coefficient | Robust SE | 95% CI | z-value | P-value |
|---|---|---|---|---|---|
| VABSCOM IQ | 0.36 | 0.0781 | 0.21–0.51 | 4.65 | <0.0001 |
| 0.40 | 0.0964 | 0.21–0.58 | 4.16 | <0.0001 |
- VABSCOM, Vineland Communication Score.
Hence, the two approaches came to the same conclusion. The estimates of familial aggregation of VABSCOM and IQbetween sibs remain relatively high even after removing the variability due to the covariates and despite the high correlation between the two measures within affected subjects (r = 0.70). Since including the covariates does not eliminate the familial aggregation pattern; IQ and VABSCOM appear to have largely distinct genetic foundations (as in Fig. 1a).
CONCLUSION
The results indicate that there are at least two familial LOF dimensions in the PDDs; non-verbal IQ and language. Even though these are highly correlated with each other within a population of affected individuals, the familial aggregation of one seems to be largely independent of the other. Presumably the high-observed correlation between these dimensions within a subject is due to the measures themselves which rely to some extent on a common trait of general cognitive ability. Although the magnitude of the familial estimates look modest, it is worthwhile pointing out that the ICC estimates we obtained make biological sense; they are roughly half what Kolevzon et al. [2004] obtained with PDD MZ twins on language skills. In essence, the data seem to support the model presented in Figure 1a; that the genetic mechanism underlying one dimension (non-verbal IQ) is largely independent of the genetic mechanisms underlying the second dimension (language skills). Of course, this conclusion is based on sibpair data not twin data, so the assumption that the familial effects reflect underlying genetic mechanisms remains to be proven. However, there are no known factors that account for familial aggregation other than genetic factors [Newschaffer et al., 2002] though environmental effects may interact with genetic susceptibility, so the assumption may be a safe one at this point in time. It is possible that children from the same family would receive similar types of early intervention which have been shown to influence IQ and language [Smith et al., 2000]. However, how this would make sibpairs more alike and so bias the results is not clear. It is also important to point out that the familial aggregation reported among affected family members is not driven by the mechanisms that account for familial resemblance in IQ and communication skills among non-affected family members. In a previous analysis, we have shown that the correlation between parents and unaffected sibs on cognitive measures is greater (and similar to that seen in typical families) than the correlation observed between parents and affected children, which was low and non-significant [see also Fombonne et al., 1997; Folstein et al., 1999; Szatmari et al., 2003].
The strengths of the study include the sampling of MPX subjects, the ascertainment scheme which ensured that a sample with wide variation in LOF and type of PDD would be included, the blind assessments, and the extensive diagnostic protocol. This study also has some limitations that should be kept in mind. The number of sibpairs was relatively small and thus the confidence intervals around the estimates of familial aggregation may be large. However, the estimates are very similar to those obtained by Silverman et al. [2002] and Spiker et al. [2002]. It is also true that the same informant reported on both children using the VABS. Though this potential rater bias would have no impact on the non-verbal IQ measure. It was our impression, however, that, if anything, parents tended to exaggerate the differences between affected children on the VABS rather than see them as more similar. More importantly, the coding of the VABS asks for observable behavior that may overcome this potential bias in reporting. It is possible however that the two assessments are administered in different ways and that this might account for the lack of one co-variate reducing the ICC of the other. It is also true that the age range of the subjects was quite large and the Leiter and the VABSCOM may not be measuring the same concept over the entire age range, particularly for a child with autism who may have unusual skills in one particular cognitive area. These are all potentially complicated measurement issues when testing subjects with autism over a wide range of abilities and ages but how this might influence the results is unclear, especially since we used age as a covariate. We would once again anticipate that such examples of potential measurement error would reduce the observed correlation rather than bias it upwards. It will be important to attempt to replicate these results not only with a different sample but also using different instruments measuring the same constructs and the same types of instruments (structured or parent administered) measuring different constructs.
These results have two important implications. The first is that it may be possible now to decompose the autism phenotype into simpler dimensions, some of which are familial within sibpairs. The PDD phenotype is first differentiated into a dimension that measures LOF and one that defines autistic symptoms. We can now further decompose the LOF phenotype into non-verbal IQ and communication/language skills which may represent more genetically informative phenotypes for linkage and association studies. It would be helpful, as a result, for genetic studies to report these separately when commenting on LOF.
The dimensions of non-verbal IQ and language can be used in two ways; as quantitative traits or as covariates. It may be that the genes that increase risk for autism are not the same genes that influence variation in non-verbal IQ or language in that disorder. Identifying genes that influence dimensions in a quantitative trait linkage analysis may be an extremely important avenue of investigation as it may be easier to identify genes associated with a simpler dimension than genes that increase risk for a complex phenotype such as autism. Indeed, we have shown that polymorphisms in the MAO and DBH genes in the mother (and possibly in the fetus) influence non-verbal IQ in a substantial way [Jones et al., 2004] but do not increase risk for autism. Several studies have also shown that using communication as a quantitative trait, increases the linkage signals at several loci [Alarcon et al., 2002; Chen et al., 2006].
In addition, these dimensions can be used as covariates in linkage analysis. This can be done through stratification, ordered subset analysis [Hauser et al., 2004], and mixture models [Devlin et al., 2002]. For example, sibships could be stratified into at least two subgroups prior to analysis using measures of non-verbal IQ. Isolating sibpairs where both have a low or high non-verbal IQ score may make the sibpairs more genetically homogeneous. In fact, focusing on the high functioning sibpairs may be quite useful. The point is not that the low functioning sibpairs are not familial. They may well be, however, the autism in these lower functioning sibpairs may be due to the presence of several different mental retardation syndromes, which are still familial, but are likely to be much more heterogeneous. Language scores could be used in a similar way to identify families where both affected sibs have language impairment and where unaffected relatives also segregate difficulties in language development. Several studies have reported promising results using this strategy [Buxbaum et al., 2001; Shao et al., 2002; Nurmi et al., 2003]. In general, the results of our analysis indicate that it may be useful to further explore the possibility of decomposing the autism phenotype into simpler but still familial dimensions and focus on these for genetic studies.
Acknowledgements
Dr. Szatmari and Dr. Zwaigenbaum were supported by Fellowship awards from the Ontario Mental Health Foundation. Dr. Roy and Dr. Mérette were supported by scientist awards from the Fonds de la Recherche en Sante du Quebec. We would like to thank the children and the families who participated in the study.