Functional COMT variant predicts response to high dose pyridoxine in Parkinson's disease
Abstract
Pyridoxal-5-phosphate, the biological active form of pyridoxine, is a cofactor for dopa-decarboxylase (DDC) enzyme. Pyridoxine may augment the conversion of levodopa to dopamine in the periphery and therefore decrease availability of levodopa to the brain. However, this effect can be negated in the presence of a DDC inhibitor, which potentiates plasma levodopa level. A single nucleotide polymorphism at the nucleotide 1947 in the catechol-O-methyltransferase (COMT) gene encodes the high (COMTH) and low activity (COMTL) forms of the enzyme. In this study, we examined the effect of the COMTL allele on the clinical response to pyridoxine in Parkinson's disease (PD) patients. PD patients who were on stable and optimized dose of levodopa were included in this study. Their mean motor and activities of living score improved after high dose pyridoxine (P = 0.09, P = 0.04), and worsened after a washout period (P = 0.005, P = 0.001). Using a multivariate model, the presence of the COMTL allele predicted response to pyridoxine, with the best outcome observed in COMTL/L homozygotes. Our observational study suggests that the status the functional COMTL variant may be potentially useful to select PD patients for high dose pyridoxine therapy. © 2005 Wiley-Liss, Inc.
INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative condition that predominantly affects the elderly [Jankovic, 2000]. Both genetic and enviromental factors may modulate the risk of PD [Tan et al., 2003, 2004; Lee et al., 2004]. Levodopa in combination with a dopa-decarboxylase (DDC) inhibitor remains the cornerstone of therapy. However, medical treatment frequently has to be individualized [Jankovic, 2000]. The relationship between pyridoxine and plasma levodopa levels has been investigated more than three decades ago [Duvoisin et al., 1969; Klawans et al., 1971; Yahr and Duvoisin, 1972; Fahn, 1974; Mars, 1974, 1975]. Pyridoxal-5-phosphate, the biological active form of pyridoxine, is a cofactor for DDC. Pyridoxine may augment the conversion of levodopa to dopamine in the periphery and therefore decrease availability of levodopa to the brain, with an increase in homovanillic acid synthesis. But this effect can be negated in the presence of a DDC inhibitor, which potentiates plasma levodopa level [Fahn, 1974; Mars, 1974, 1975] (Fig. 1).
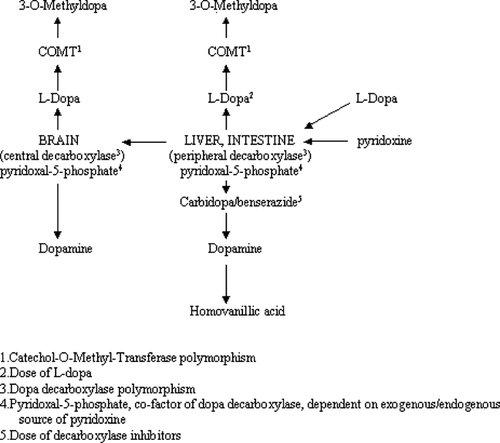
Potential clinical genetic factors affecting availability of dopamine to brain.
The enhancement of peripheral levodopa degradation by pyridoxine and the action of DDC inhibitors can be variable [Mars, 1974, 1975]. It is not clear in an individual PD patient, what pyridoxine and DDC inhibitor dose would affect motor function. Previous biochemical studies have focused on metabolic interaction of pyridoxine and levodopa with oral or intravenous and intramuscular injections of 25–100 mg of pyridoxine [Duvoisin et al., 1969; Klawans et al., 1971; Yahr and Duvoisin, 1972; Fahn, 1974; Mars, 1974, 1975]. However such route or dose of pyridoxine may not truly reflect prescriptions in clinical practice.
A single nucleotide polymorphism at the nucleotide 1947 in the catechol-O-methyltransferase (COMT) gene encodes the high (COMTH) and low activity (COMTL) forms of the enzyme, with a valine substitution for methionine at the 158/108 locus in the peptide sequence. The COMTL allele results in a heat-labile protein with a fourfold reduction in enzymatic activity [Mannisto and Kaakkola, 1999]. This variant has been demonstrated to influence dopamine regulation in the human brain by their variable conversion of levodopa to 3-O-methyldopa [Akil et al., 2003]. It may be associated with risk of PD [Tan et al., 2000; Wu et al., 2001].
In this study, we examined; (1) the effect of high dose pyridoxine on the motor function during the “on” state in PD patients, and (2) the effect of the COMTL allele on the clinical response to pyridoxine.
MATERIALS AND METHODS
The trial was designed as a prospective, patient-controlled, crossover study. Consecutive PD patients were recruited and evaluated by a movement disorder specialist in the outpatient clinic. PD patients who were on stable and optimized dose of levodopa (levodopa and DDC inhibitors) for at least 3 months prior to study were included. The exclusion criteria were; (1) patients who were taking vitamin B or other vitamin preparations or drugs that can interact with pyrixodine (such as phenytoin, monoamine oxidase inhibitors, etc), (2) advanced PD (Hoehn & Yahr stage 5), (3) patients who suffered from poor nutrition, malabsorption, or had gut surgery, (4) patients with debilitating and life threatening systematic illness, (5) patients with allergy to any component of Neuroforte®, and (6) unreliable patients.
Motor Examination
All patients were examined during the “on” state for their baseline motor function and the activities of daily living (ADL) using the Unified PD Rating Scale. “On” was defined as the time when both patients and investigator agreed that the levodopa response was optimal, and this happened usually 2–3 hours after a levodopa dose. They were instructed to take one tablet of Neuroforte® (Merck, Germany) (each tablet contains 200 mg of pyridoxine, 100 mg thiamine, and 200 mcg of cyanocobalamin) twice daily (at the same time as Madopar® or Sinemet®) for 4 weeks. This was equivalent to 400 mg pyridoxine daily.
The patients were examined 4 weeks later during the “on” state, similar as examination at baseline. After a 2 weeks washout period of pyridoxine, they were re-examined again following similar procedures. The dosages of their levodopa and other PD drugs were kept constant throughout.
Dietary Intake of Pyridoxine
The patients were evaluated on their dietary habits to estimate the amount of pyridoxine obtained from their daily diet. The dietary habits of a list of 21 food items that contained significant amounts of pyridoxine and frequently consumed by our local population were investigated. Subjects were interviewed regarding the food items consumed daily over a 4 weeks period. We calculated the amount of pyridoxine consumed in milligrams by each subject over a course of 1 month based on the definitions and recommendations of the USDA National Nutrient Database for Standard Reference (http://www.nal.usda.gov/fnic/foodcomp/Data/SR17/wtrank/wt_rank.html).
Genetic Analysis of COMT and DDC Genes
Blood was taken from study subjects as part of a larger study on genetic susceptibility in PD. For COMT, the polymerase chain reaction (PCR) was carried out using primer pair of F-5′-TCGTGGACGCCGTGATTCAGG-3′ and R-5′-AGGTCTGACAACGGGTCAGGC-3′ under conditions previously described [Wu et al., 2001; Akil et al., 2003]. The PCR products were digested with NlaIII and the COMTH (Val-108) allele, and COMTL (Met-108) allele were differentiated on gel electrophoresis.
A genetic polymorphism (4 bp deletion in exon1, of unknown significance) of the DDC enzyme (Fig. 1) was examined as a control. Genotyping was carried out using primers F-5′-GAG GGA TGC TGC TCA GTA AA-3′, R-5′-ATC CAG AGA GCT GGA CGC-3′ under previously described conditions [Borglum et al., 1999]. Informed consent was taken from every study subject for both the clinical and genetic analysis, and the institution ethics committee approved the work.
Blinded Examination/Analysis
Two groups of investigators conducted the clinical assessment and genetic analysis independently, and were blinded to the clinical and genetic data available to each group. A third group of investigators carried out the analysis of the clinical and genetic data at the end of the study period.
Statistical Analysis
Two main outcome measures were evaluated; change in ADL and change in motor function score from baseline value. MANOVA was carried to find out the independent predictors for the ADL change and motor function change when the two outcomes were considered simultaneously. MANOVA works by creating a composite measure of the two dependent variables. The factors and covariates included in the MANOVA model were COMT polymorphism, DDC polymorphism, dietary intake of pyridoxine, age, gender, stage and duration of disease, and dose of levodopa.
RESULTS
Out of 45 patients who participated, we found 6 protocol violators (5 skipped the dose more than once, and 1 defaulted follow-up). Thirty-nine patients were finally analyzed comprising 24 men (61.5%) and 15 women (38.5%) with mean age of 69.9 (±7.6), and mean duration of disease 5.9 years (SD ± 3.2). The majority was of H&Y stage 2–2.5 (61.5%) (range 2–4). The mean total daily dose of levodopa was 421.5 mg (SD ± 226.2) and the mean dietary intake of pyridoxine was 7.37 mg/week (SD ± 2.78).
The mean baseline ADL score was 13.4 (SD ± 4.8), which decreased to 12.9 (SD ± 4.1) after the patients took pyridoxine for 4 weeks (P = 0.09) and increased to 13.7 (SD ± 4.7) (P = 0.005) after the washout period. The mean motor score was 37.9 (SD ± 8.3), which improved to 36.7 (SD ± 8.2) after pyridoxine for 4 weeks (P = 0.04) and worsened to 38.7 (SD ± 8.5) after the washout period (P = 0.001).
The COMTL allele was present in 26/78 (33.3%) of patients, with COMTL/H heterozygotes accounting for 20/39 (51.3%) followed by wild type 16/39 (41.0%) and COMTL/L homozygotes 3/39 (7.7%). The multivariate Pillai's Trace statistics from MANOVA showed that the COMTL variant significantly differed in terms of the composite measure of ADL change and motor function change (F(4,52) = 2.943, P = 0.029). The COMTL was significantly associated with motor function improvement (F(2,26) = 6.788, P = 0.004) (Table I). The dose of levodopa weakly influenced ADL change (F(1,26) = 4.894, P = 0.036). DDC polymorphism, gender, age, stage of disease, dietary intake of pyridoxine, and duration of PD were not significant predictors (Table I).
| Factor | Dependent variable | Hypothesis df | F | P-value |
|---|---|---|---|---|
| Gender (male/female) | ADL change | 1 | 3.009 | 0.095 |
| Motor function change | 1 | 0.685 | 0.415 | |
| Stage of disease (H & Y 1–4) | ADL change | 3 | 1.282 | 0.301 |
| Motor function change | 3 | 1.113 | 0.362 | |
| COMT polymorphism (high/low activity) | ADL change | 2 | 0.349 | 0.709 |
| Motor function change | 2 | 6.788 | 0.004 | |
| DDC polymorphism (deletion/no deletion) | ADL change | 2 | 0.137 | 0.872 |
| Motor function change | 2 | 2.333 | 0.117 | |
| Dietary pyridoxine dose (mg/week) | ADL change | 1 | 0.001 | 0.973 |
| Motor function change | 1 | 0.085 | 0.773 | |
| Age (years) | ADL change | 1 | 0.659 | 0.424 |
| Motor function change | 1 | 2.382 | 0.135 | |
| Duration of disease (years) | ADL change | 1 | 1.512 | 0.230 |
| Motor function change | 1 | 0.110 | 0.743 | |
| Dose of levodopa (mg/day) | ADL change | 1 | 4.894 | 0.036 |
| Motor function change | 1 | 0.327 | 0.573 |
Simple a priori contrast testing with t statistics revealed that COMTL/L homozygotes demonstrated a significantly drop (from baseline) in motor function score than wild type (t = −3.628, P = 0.001). The mean change in motor score (after-before neuroforte) was −8.082 (SD ± 6.0) for COMTL/L homozygotes, −1.961 (SD ± 2.4) for COMTL/H heterozygotes, and +0.338 (SD ± 3.1) for the wild type.
DISCUSSION
Pyridoxine is a constituent of most multivitamin preparations available over the pharmacy's counter. Manufacturers' information sheet instructs caution if levodopa is to be prescribed. Vitamin B preparations are frequently prescribed for, or self-administered by PD patients.
We examined the effect of a widely available vitamin B formulation that contains a high dose of vitamin B6 (200 mg/capsule) in a select group of well nourished PD patients who were on optimized levodopa regime. We demonstrated an improvement of the mean motor score of 1.2 after 1 month of pyridoxine and a deterioration of two points after the washout period. Similar findings were found in the ADL score. In the multivariate analysis, the presence of the functional COMTL allele but not the other studied variables predicted improvement in motor function. Further analysis of the COMT genotypes demonstrated the greatest improvement in patients who were COMTL/L homozygotes. Using a DDC polymorphism of unknown function as a genetic control, such an association was not demonstrated.
The probability of a false positive finding was low for the following reasons; (1) the genetic and clinical analysis was carried out by investigators independently, (2) the strict inclusion and exclusion criteria restricted confounding variables, (3) the statistic (P = 0.004) observed for the COMT allele in the multivariate model was robust, (4) the graded improvement demonstrated in the COMT genotypes, and (5) the lack of association of the DDC polymorphism in the control arm. Importantly our findings make biological sense. In patients with COMTL allele, the conversion of levodopa to 3-0-methyldopa is reduced, allowing more levodopa to be converted to dopamine. High dose pyridoxine (a co-factor for brain decarboxylase) facilitates the metabolism of levodopa to dopamine in the brain. In the periphery, the presence of DDC inhibitors negates the action of pyridoxine and more of the levodopa enters the brain in those with the COMTL allele (Fig. 1). Hence the overall effect of taking high dose pyridoxine is greatly facilitated in the brains of those with the COMTL allele.
Our observational study has some limitations. The sample size did not allow us to conduct a more effective subset comparison between those with different genotypes, though the COMTL/L homozygotes showed the greatest improvement, followed by the COMTL/H heterozygotes and those with the wild type. However, based on a motor function change of eight points (with SD of 3), our sample size has more than 95% power to detect the observed difference between the COMTL/L homozygotes and wild type at α = 0.01. Similarly it has 80% power to detect a difference of six points (SD of 3) between COMTL/H heterozygotes and wild type at α = 0.01. In addition, the multivariate analysis demonstrated robust association of the COMTL allele with clinical improvement. It is not clear whether vitamins B1 and B12, minor constituents of Neuroforte® have any complimentary role in the outcome.
In conclusion, our study suggests that the status of a functional COMTL variant may be potentially useful to select PD patients for high dose pyridoxine (at 400 mg/day) therapy. Further double blind controlled studies are warranted to further investigate the utility of high dose pyridoxine as a potential supplement to the PD therapeutic regime.
Acknowledgements
The authors thank their PD patients and caregivers for raising the queries that led to formulation of the study objectives. No financial assistance was received from any pharmaceutical companies. We also thank Eileen Lim, Ian Lim, and Valerie Tan for their assistance. We have no competing interest to declare. Authors Tan, Zhao, Cheah, Fook-Chong conceived, and planned the study. All authors helped in the clinical and genetic analysis, and contributed to the writing of the manuscript.




