Whole Blood Multi-OMIC Analysis Is Effective in Clinical Interpretation of Splicing Aberrations in PLOD1-Related Kyphoscoliotic Ehlers-Danlos Syndrome
Funding: This work was supported by Ministero della Salute. Fondazione Telethon. Giovanni Armenise-Harvard Foundation. Ministero dell'Istruzione, dell'Università e della Ricerca.
ABSTRACT
Kyphoscoliotic Ehlers-Danlos syndrome (kEDS) Type 1 is a rare hereditary connective tissue disorder due to biallelic deleterious variants in PLOD1 and is mainly characterized by hypotonia, congenital kyphoscoliosis, eye fragility, hyperextensible skin, Marfanoid habitus, and joint hypermobility. In PLOD1-kEDS, deleterious variants are typically loss-of-function alleles. Therefore, the identification of private non-canonical splicing variants might deserve functional studies to refine their clinical interpretation. In a 7-year-old boy with congenital hypotonia, kyphoscoliosis, joint hypermobility, and arachnodactyly, exome sequencing revealed the homozygous variant c.1756-13C > A in PLOD1. This variant was previously annotated in public databases with conflicting pathogenicity criteria. In contrast to other EDS subtypes whose causative gene is not expressed in peripheral lymphocytes, whole blood RNA sequencing demonstrated a deleterious effect of the identified variant, which resulted in the incorporation of 11 intronic nucleotides and the generation of a premature stop codon. In this work, multi-OMIC analysis on peripheral blood was a rapid and reliable tool to clinically characterize a non-canonical splice site variant in PLOD1-kEDS.
1 Introduction
Kyphoscoliotic Ehlers-Danlos syndrome (kEDS, OMIM 225400) is an autosomal recessive subtype of Ehlers-Danlos syndrome (EDS) characterized by congenital non-progressive hypotonia, congenital and/or early-onset kyphoscoliosis, generalized joint hypermobility and related musculoskeletal manifestations, Marfanoid habitus, reduced bone mass, skin hyperextensibility and fragility, easy bruising, and arterial ruptures (Beighton et al. 1998; Rohrbach and Giunta 1993–2025). kEDS includes two clinical-molecular entities, which are type 1 kEDS due to biallelic deleterious variants in the gene encoding lysyl-hydroxylase-1 (LH1, or procollagen-lysine,2-oxoglutarate 5-dioxygenase 1; PLOD1) (PLOD1-kEDS) and type 2 kEDS caused by inherited alterations in the FKBP14 gene (Malfait et al. 2017; Lim et al. 2019). LH1 is a post-translational modification enzyme that is essential for the formation of intra- and intermolecular collagen cross-links. LH1 deficiency results in under-hydroxylation of collagen lysyl residues, leading to impaired cross-link formation and consequent mechanical instability of the affected tissues (Rohrbach et al. 2011). Eye fragility, if present, and an abnormal pattern of lysyl and hydroxylysyl pyridinoline cross-links in the urine, if assessed (Malfait et al. 2017; Rohrbach et al. 2011), may differentiate PLOD1-kEDS from FKBP14-kEDS on clinical grounds.
To date, the commonest deleterious variant in PLOD1 is an 8.9 kb duplication generated by an Alu-Alu recombination between Introns 9 and 16 (Brady et al. 2017). The other variants most frequently annotated as disease-causing in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar) and LOVD (https://databases.lovd.nl/shared/genes) are frameshift, nonsense, and ± 1,2 donor/acceptor splice site. In this context, the characterization of private variants that potentially affect splicing but do not fall within canonical splice sites is critical for the effective application of the American College of Medical Genetics and Genomics/Association for Molecular Pathology (ACMG/AMP) criteria (Walker et al. 2023; Richards et al. 2015). RNA sequencing (RNA-seq) is feasible to resolve undiagnosed cases by determining the functional transcriptional consequences of splice site, intronic, and deep intronic variants that remain classified as unknown significance or may otherwise be missed by traditional genome sequencing (Kremer et al. 2017).
Here, we report a 7-year-old boy carrying the homozygous PLOD1 c.1756-13C > A variant, which was subsequently characterized by RNA-seq on peripheral blood for clinical use.
2 Patients and Methods
The proband (Individual II,2) and his family were enrolled at Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy. This study is in accordance with the 1984 Helsinki declaration and subsequent revisions and received IRB approval at Fondazione IRCCS-Casa Sollievo della Sofferenza (approval no. 2023/45/CE). The parents' proband signed written informed consent for the publication of clinical, pictorial, and molecular data.
2.1 Exome Sequencing (ES)
DNA was extracted from peripheral blood by using the QIAsymphony AS instrument (Qiagen, Hilden, Germany). Gene-agnostic exome sequencing (ES) was carried out on samples obtained from the proband and parents for a sporadic case of unclassified neonatal hypotonia (HP:0001319) and kyphoscoliosis (HP:0002751). ES was executed through Illumina DNA Prep with the Exome 2.0 Plus Enrichment kit and analyzed by massive-parallel sequencing with Illumina NovaSeq 6000 (PE 2 150, Illumina Inc., San Diego, CA, USA). We performed data analysis through Illumina DRAGEN version 3.9.5 for reads alignment and variant calling while annotation, filtering, and visualization of variants were performed using the Illumina TruSight Software Suite version 2.6. Variants were prioritized by assuming a rare monogenic disorder with sporadic occurrence in a male individual (i.e., dominant de novo, autosomal recessive or X-linked recessive patterns). Variants were filtered according to the following criteria: (i) variants absent or very rare (variant allelic fraction < 0.0005 for dominant/X-linked genes and < 0.01 for autosomal recessive genes) in GnomAD; (ii) null alleles (nonsense, frameshift, ±1,2 D/A sites) in genes prone to loss-of-function; (iii) variants previously known as deleterious in PubMed or public databases (i.e., ClinVar); (iii) inframe indels in non-repetitive sequences; (iv) missense variants predicted deleterious in silico (REVEL score > 0.664 and/or CADD score > 25.3); synonym, intronic and missense variants predicted to alter the splicing (SpliceAI > 0.2).
Extended cascade testing in the unaffected proband's sister of the selected variant (see Section 3) was performed by Sanger sequencing using the following primers to amplify exon 17 of PLOD1: 5′-AAGCCTGTTCAGTTTTGTCATGTAA-3′ (forward) and 5′-CCCTCAGTCCCTCTACCTCATGC-3′ (reverse).
2.2 In Silico Prediction
In silico analysis of the c.1756-13C > A intronic variant was evaluated by four splicing prediction programs (SpliceSite-Finder-like, MaxEntScan, NNSPLICE and Human Splicing Finder) included in the Alamut Software suite (Interactive Biosoftware, Rouen, FR) and the Database Splicing Consensus Single Nucleotide Variant program (dbscSNV) incorporated in the Franklin platform (https://franklin.genoox.com/clinical-db/home), which provides the splicing prediction value for the investigated variant called SpliceAI (Jaganathan et al. 2019). NMDEscPredictor tool was used to predict whether a given frameshifting indel activates or escapes the nonsense mediated mRNA decay based on the relative location of the variant within the gene (https://nmdprediction.shinyapps.io/nmdescpredictor/).
2.3 RNA Sequencing (RNA-Seq)
Independent blood drawn was collected, and individual samples were harvested in Tempus Blood RNA Tube (Thermo Fisher Scientific, Waltham, MA, USA). RNA was extracted using custom RNA extraction core services using paramagnetic beads technology (Fondazione Telethon spin-off—Negedia s.r.l.). Total RNA was quantified using Qubit 4.0 fluorimetric Assay (Thermo Fisher Scientific, Waltham, MA, USA). Libraries were prepared from 500 to 1000 ng of total RNA using the NEGEDIA Full Length mRNA-seq sequencing service (Negedia s.r.l.), which included stranded library preparation, quality assessment, and sequencing on a NovaSeq 6000 sequencing system using a paired-end, 150 cycle strategy (Illumina Inc., San Diego, CA, USA). FASTQ reads were analyzed with Negedia s.r.l. proprietary full-length mRNA-seq pipeline (v1.0), which involves quality control, alignment to the reference hg38 human genome, and counting steps (Smith et al. 2017; Krueger et al. 2023; Dobin et al. 2013; Liao et al. 2014). IGV was applied to visualize the BAM file's exon coverage, positional variants, and sashimi plots based on sequences mapping across exon junctions (split reads).
2.4 Reverse Transcription PCR Analysis (RT-PCR)
The predicted in silico effect and data obtained from RNA-seq was further investigated by reverse transcription PCR (RT-PCR). Total RNAs from lymphocyte fractions isolated from the peripheral blood of patients and a pool of healthy individuals were extracted using PAXgene blood RNA kit (Qiagen, Hilden, Germany). Extracted RNAs were treated with RNase-free DNase (Qiagen, Tübingen, Germany) and quantified by Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA), and reverse-transcribed with the QuantiTect Reverse Transcription Kit (Qiagen, Tübingen, Germany), according to the manufacturer's protocol. cDNA was PCR amplified using primers designed to amplify exons 15–18 of PLOD1. The primers were checked by BLAST and BLAT against the human genome to ensure specificity; 5′-CCAGAACTACACCAAAGCCCTG-3′ (forward) and 5′-GGACGACAAAGGAGGCATCATG-3′ (reverse). PCR products were examined by 1% agarose gel. The amplified product was subsequently purified by using ExoSAP-IT PCR Product Cleanup Reagent (Thermo Fisher Scientific, Waltham, MA, USA) and sequenced by using BigDye Terminator v1.1 sequencing kit (Thermo Fisher Scientific, Waltham, MA, USA). The fragment obtained was purified using DyeEx plates (Qiagen, Tübingen, Germany), resolved on ABI Prism 3130 Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed using the Sequencher software (Gene Codes, Ann Arbor, MI, USA).
3 Results
3.1 Clinical Findings
The proband is the second child of Senegalese unaffected and consanguineous parents (first-degree cousins). He was born at term (40 weeks) by spontaneous delivery and a pregnancy complicated by oligohydramnios of unknown cause. Birth parameters were length 52.3 cm (45th centile), weight 3195 g (16th centile), head circumference 33.4 cm (4th centile) and Apgar score 8(1′)/10(5′). Examination at birth disclosed congenital hypotonia, multiple peripheral joint contractures, and hip dysplasia on the left. For these findings, the patient underwent physical therapy and cast application, with resolution of the contractures on hands and feet and hip dysplasia. Though improved, muscle hypotonia persisted over the years. Transfontanellar ultrasound at birth disclosed congestion of the germinal matrix and hyperechogenicity of the thalamus and basal ganglia bilaterally. These findings disappeared at brain magnetic resonance imaging performed at 4 years of age. The boy said his first words at 24 months and walked alone at 4 years due to muscle hypotonia. He also developed kyphoscoliosis, for which he underwent physical therapy and the application of an orthopedic brace. At 2 years, the patient needed surgical repair of umbilical hernia and bilateral cryptorchidism. At 5 years 2 months, the patient was height 112 cm, weighed 16.9 kg, and had a head circumference of 50.5 cm. He displayed a dolichocephalic skull with a prominent occiput, prominence of the metopic suture (without evidence of true craniosynostosis at imaging), bilateral epicanthal folds, depressed nasal bridge, marked kyphosis with intrarotated shoulders, bilateral elbow limitation, slender fingers with positive thumb and wrist signs bilaterally (arachnodactyly), hyperextensibility of the small joints of hands and feet (Beighton score 4/9), severe flatfeet, soft and hyperextensible skin, and a single keloid on the left leg (Figure 1A). Physical examination at 7 years disclosed height 133.2 cm, arm span 134.2 cm, and arm span/height ratio of 1.008 (normal). Review of clinical data after molecular results allowed recognition of a phenotype consistent with mild kEDS Type 1.
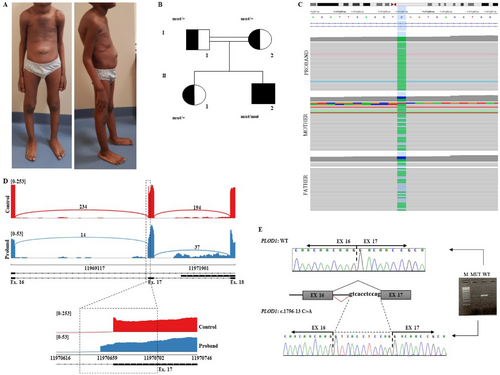
3.2 Multi-OMIC Findings
ES in Individual II,2 revealed the homozygous splicing variant c.1756-13C > A at position Chr1(GRCh38):g.11970657 located in intron 16 of PLOD1 (GenBank accession number: NM_000302.4, Uniprot: Q02809). Not any other strong candidate was identified according to the available clinical data. The c.1756-13C > A variant was absent in major databases [dbSNP (https://www.ncbi.nlm.nih.gov/snp/), 1000 Genomes (https://www.internationalgenome.org/), and gnomAD (https://gnomad.broadinstitute.org/)] and was previously reported a single instance as “variant of uncertain significance” and “likely pathogenic” in the ClinVar (Variation ID: 2441983) and LOVD (Individual ID: 00434996), respectively. In a previous publication, describing a severe case of PLOD1-related kEDS, the same variant was reported in trans with a null allele but without any functional study demonstrating its effect on splicing (Foy et al. 2023). Both healthy parents and the unaffected sister of our Individual II,2 were heterozygous carriers (Figure 1B,C). The SpliceAI score was 0.99, suggesting a highly likely splicing effect for the variant. In silico analysis [NetGene2 (https://services.healthtech.dtu.dk/services/NetGene2-2.42/) and Fruitfly (https://www.fruitfly.org/seq_tools/splice.html)] predicted that the variant would likely mediate the use of an alternative cryptic splice site nearby.
As PLOD1 is physiologically expressed in the blood tissue, we assessed the effect of the c.1756-13C > A at the transcriptional level using a full-length mRNA-seq analysis on peripheral blood samples of Individual II,2 compared to our control dataset including four samples. The analysis showed that the intronic change causes the activation of a cryptic splicing acceptor site in intron 16 of the PLOD1 gene, resulting in the non-in-frame retention of 11 intronic nucleotides (r.1755_1756insGTCACCTCCAG) in the gene transcript (Figure 1D). This event is predicted to result in a frameshift which leads to a truncated LH1 lacking the C-terminal residues [p.(Asp586Valfs*22)]. The aberrantly spliced transcript is not present in the expressed sequence tag database (EST, https://www.ncbi.nlm.nih.gov/dbEST/index.html). The inclusion of 11 intronic nucleotides in the mutant PLOD1 mature RNA was confirmed by RT-PCR (Figure 1E). The generated PTC was located in exon 16 at a distance of more than 50 nucleotides upstream of the 3′-most exon-exon junction, a condition that generally triggers NMD. The NMDEscPredictor tool predicted that the aberrant transcript activated the NMD. The lower coverage at RNA-seq shown in the proband sample compared to controls could be explained by NMD (Figure 1D). Additional functional and clinical information allowed us to definitively classify the variant as “probably pathogenic”: PVS1 (Strength)_Strong, PM2_Moderate, PM3_Supporting, and PP4_Supporting.
4 Discussion
Here, we report a 7-year-old boy with kEDS due to the homozygous c.1756-13C > A variant in PLOD1. Whole blood RNA-seq and RT-PCR demonstrated that the c.1756-13C > A variant alters the splicing by activating a novel acceptor splice site, which induces the expression of an aberrant transcript containing a PTC. Our study showed that the combined whole blood ES/RNA-seq analysis is effective to solve cases of kEDS due to private or poorly characterized non-canonical splice variants.
Aberrant splicing is known to be a major cause of Mendelian diseases (Kremer et al. 2017; Wagner et al. 2023), but it is not always possible to predict the consequences of splicing defects from the genome alone, as splicing is a complex mechanism involving a set of cis-regulatory elements that are not fully understood (Kremer et al. 2017). To overcome these limitations, RNA-seq has been adopted in the last years to detect the effect of specific variants of uncertain significance on the splicing process and to identify de novo aberrant splicing events across the transcriptome. For example, this approach was effective in documenting multiple splicing defects based on single gene studies, such as skipping of multiple exons (Muntoni et al. 2003) and creation of a new exon by a deep intronic variant (Gonorazky et al. 2019) in DMD, intron retention caused by a 5′ splice site variant in LMNA (Morel et al. 2006), and the generation of multiple aberrant transcripts by a single intronic change in SRCAP (Morlino et al. 2024).
A major limitation to the clinical application of RNA-seq is the expression constraints of most genes due to their tissue specificity and/or transcript variability due to tissue-specific alternative splicing. Accordingly, the availability of expressing tissue(s) to sampling is crucial for the effective application of RNA-seq. For example, skin biopsy has been demonstrated to be much more representative than peripheral blood for hereditary neuromuscular disorders (Gonorazky et al. 2019). Such a perspective is reasonable also for EDSs according to their underlying pathomechanisms (Malfait et al. 2020). Accordingly, RNA-seq was applied in PLOD1-kEDS and FKBP14-kEDS patient-derived skin fibroblasts to explore, on a research basis, their molecular pathogenesis by highlighting distinguishable transcriptional patterns (Lim et al. 2019). Gene ontology enrichment analysis revealed differential expression of genes encoding extracellular matrix components, and several pathways including ear development, vascular modulation, endoplasmic reticulum stress, bone remodeling, and protein trafficking were found to be differentially represented between these two disorders (Lim et al. 2019). However, PLOD1 is also well expressed in peripheral blood compared to FKBP14 for kEDS Type 2 and many other EDS-associated genes such as COL1A1, COL1A2, COL5A1, and COL3A1. This implies that whole blood RNA-seq may be used to investigate the transcriptional patterns of PLOD1 to integrate DNA analysis for clinical use.
Our work documents for the first time the reliable use of whole blood RNA-seq to demonstrate the deleteriousness of non-canonical splicing variants in PLOD1-related kEDS without proceeding with a skin biopsy, a procedure that is questioned in specific real-world circumstances.
Author Contributions
F.R. conceptualization, editing, writing – original draft, writing – review and editing. C.D. clinical data collection and photo collection. E.D.M. RNA-seq and bioinformatic analysis. L.P. ES analysis. L.G. ES analysis. L.V. RNA-seq and bioinformatic analysis. M.I. ES analysis. D.C. RNA-seq and bioinformatic analysis. M.C. conceptualization, project administration, writing – review and editing. L.M. conceptualization, editing, writing – original draft, writing – review and editing. All authors read and approved the final manuscript.
Acknowledgments
We are grateful to the family who agreed to participate in this study. We also thank the Bioinformatics Core Facility of the Telethon Institute of Genetics and Medicine (TIGEM). M.C. is Institutional Representative of ERN-Skin and ERN-ReCONNET at Fondazione IRCCS-Casa Sollievo della Sofferenza (Italy).
Ethics Statement
This study was performed in line with the principles of the Declaration of Helsinki and its following modifications and received IRB approval at Fondazione IRCCS-Casa Sollievo della Sofferenza, Italy (protocol no. 2023/45/CE).
Consent
Family signed written informed consent for the publication of clinical and molecular data.
Conflicts of Interest
The authors declare no conflicts of interest. Davide Cacchiarelli is the founder, shareholder, and consultant of NEGEDIA s.r.l.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




