Tall Stature and Scoliosis Associated With a Novel Homozygous Loss-of-Function Missense Variant in NPR3
Funding: This work was supported by the Shriners of North America and the Saputo Foundation.
ABSTRACT
NPR3-related tall stature is characterized by tall stature, elongated big toes, and additional epiphyses in hand and foot bones. The condition is caused by biallelic loss-of-function variants affecting natriuretic peptide receptor 3 (NPR3). Five individuals from four different families have been reported. Here we describe three siblings with NPR3-related tall stature who were tall (height z-scores between +2.9 and + 4.9) and had markedly elongated proximal and middle phalanges. Two siblings had additional epiphyses in phalangeal and metacarpal bones. All three siblings developed scoliosis, requiring spinal fusion surgery in one individual. Lumbar spine bone mineral density appeared low considering the tall stature. Sequencing of a skeletal disorders gene panel in one sibling revealed a homozygous missense variant in NPR3 (NM_001204375.2; c.382C>T; p.Pro128Ser). Sanger sequencing demonstrated the same homozygous variant in the other siblings. In vitro functional testing in MC3T3-E1 preosteoblastic cells showed that NPR3 carrying the p.Pro128Ser variant was expressed but was retained in the endoplasmic reticulum, leading to loss of NPR3 function. In conclusion, the novel homozygous p.Pro128Ser loss-of-function variant in NPR3 led to the typical features of NPR3-related tall stature and, in addition, was associated with scoliosis. These observations expand the genotypic and phenotypic spectrum of NPR3-related tall stature.
1 Introduction
NPR3-related tall stature, also called Boudin–Mortier syndrome (OMIM # 619543), is a recently identified syndrome that is characterized by tall stature, elongated big toes and additional epiphyses (“pseudoepiphyses”) in the long bones of hands and feet (Boudin et al. 2018). Joint hyperlaxity and aortic dilatation have also been observed in some of the individuals with NPR3-related tall stature that have been reported to date (Boudin et al. 2018; Lauffer et al. 2022). Overall, the condition has clinical resemblance with Marfan syndrome.
NPR3-related tall stature is caused by biallelic loss-of-function variants affecting natriuretic peptide receptor 3 (NPR3, also called NPR-C), which together with NPR1 (NPR-A) and NPR2 (NPR-B) forms the family of transmembrane natriuretic peptide receptors (Della Corte et al. 2023) NPR2, NPR3, and one of their ligands, C-type natriuretic peptide (CNP), play a key role in the regulation of growth plate activity (Rintz et al. 2022). Activation of NPR2 by CNP leads to the differentiation and hypertrophy of growth plate chondrocytes and stimulates these cells to secrete extracellular matrix. NPR3 closely resembles NPR2 in its extracellular domain and binds CNP but acts as an inhibitor of CNP signaling.
In accordance with the contrasting effects of NPR2 and NPR3 on CNP signaling, pathogenic variants in their respective genes lead to opposite effects on growth. Loss-of-function variants in NPR2 result in short stature, whereas NPR2 gain-of-function variants give rise to tall stature (Rintz et al. 2022). Homozygous loss-of-function variants in NPR3 are also associated with tall stature, presumably because of the increased availability of CNP that signals through NPR2 (Boudin et al. 2018). The importance of NPR3 for the control of stature is also clear from genome-wide association studies, where common variants in NPR3 are consistently associated with height (Yengo et al. 2022).
Until now, only five individuals with NPR3-related tall stature from four different families have been reported (Boudin et al. 2018; Lauffer et al. 2022). In the present report, we describe three siblings with NPR3-related tall stature caused by a novel homozygous missense variant and present in vitro data to demonstrate that the variant causes loss-of-function. In addition to the previously described features of NPR3-related tall stature, severe scoliosis developed in one sibling and mild scoliosis in the two others, an observation that had not been previously reported in NPR3-related tall stature and therefore extends the phenotype associated with biallelic NPR3 loss-of-function variants.
2 Patients and Methods
2.1 Subjects
The three siblings presented here were last evaluated at the Shriners Hospitals for Children—Canada at the ages of 12, 19, and 24 years. The case study was approved by the Institutional Review Board of McGill University. Informed consent was obtained from the two adult individuals; parental informed consent was obtained for the youngest sibling. Clinical data were obtained by chart review.
2.2 Clinical Assessment
Height was measured using a Harpenden stadiometer (Holtain Limited, Crymych, UK), and weight was determined using mechanical scales (Healthometer, Bridgeview, USA). Height and weight were converted to age- and sex-specific z-scores according to the reference data published by the Centers for Disease Control and Prevention (Ogden et al. 2002).
Blood samples were obtained in the morning after an overnight fast. Serum concentrations of procollagen Type 1 amino-terminal propeptide (P1NP) and carboxy-terminal collagen crosslinks (CTX) were measured by chemiluminescence assays on an IDS-iSYS automated analyzer (Immunodiagnostics Systems Limited, UK).
2.3 Bone Densitometry
Dual-energy X-ray absorptiometry (DXA) was performed in the antero–posterior direction at the lumbar spine (L1–L4) and the total body using a Hologic QDR Discovery device (Hologic Inc., Waltham, MA, USA). Results were transformed to age- and gender-specific z-scores using reference data provided by the manufacturers.
Peripheral quantitative computed tomography (pQCT) was performed at the radius using the Stratec XCT2002 equipment (Stratec Inc., Pforzheim, Germany) and the manufacturer's software package (version CXT 6.00B). Cross-sectional measurements (2 mm slice thickness) were taken at sites corresponding to 4% (distal radius) and 65% (radius shaft) of forearm length. Results were transformed to age- and sex-specific z-scores using our published reference data (Rauch and Schoenau 2005; Rauch and Schoenau 2008).
2.4 Sequencing
Genomic DNA of one of the siblings was analyzed by next-generation sequencing (Illumina NextSeq 1000) of a Skeletal Disorders Panel. The panel included 548 genes with a confirmed association with monogenetic skeletal and connective tissue disorders (rated ‘green’ by PanelApp) (Stark et al. 2021). Exons and 20 nucleotides of surrounding intronic sequence were analyzed at the Biomedical Laboratory at Shriners Hospital for Children—Canada, which is accredited for clinical molecular diagnostic testing by the College of American Pathologists. REVEL was used as the computational tool for missense variant pathogenicity classification. This provides a score that integrates the scores of 13 individual in silico prediction tools (MutPred, FATHMM, VEST, Poly-Phen, SIFT, PROVEAN, MutationAssessor, MutationTaster, LRT, GERP, SiPhy, phyloP, and phastCons) (Ioannidis et al. 2016). A systematic analysis of prediction algorithms has shown that REVEL is one of the highest performing tools (Pejaver et al. 2022). After the NPR3 variant was identified by next-generation sequencing, the presence of the variant was confirmed in all three siblings using Sanger sequencing. Sanger sequencing of the locus was also performed in a control DNA sample (NA12878).
2.5 Cloning and Mutagenesis
A pcDNA3.1-based plasmid expressing human NPR3 (NM_001204375.2) with a C-terminal FLAG tag (clone ID OHu0962) was obtained from GenScript (Piscataway, USA). This plasmid was modified to insert a MYC tag (EQKLISEEDL) at the N-terminus just after the NPR3 signal peptide sequence. This doubly tagged plasmid was then used for site-directed mutagenesis to introduce the p.Pro128Ser variant. For comparison, NPR3 carrying a previously described variant, p.Ser148Pro, was also created (Boudin et al. 2018). The modifications were introduced using an enzyme restriction and PCR-based approach. PCR products were amplified with the high-fidelity Phusion DNA polymerase (NEB) and digested with the appropriate restriction enzymes. These were reintroduced after enzymatic restriction and ligation into the parent plasmid. All oligonucleotide sequences and restriction enzymes are available from the authors on request. The plasmids encoding placental alkaline phosphatase (PLAP)-OSTN (consisting of mouse osteocrin amino acid residues 29 to 130) and PLAP-CNP (consisting of mouse CNP amino acid residues 74 to 126) fusion secreted proteins were obtained from conditioned media of transfected HEK293 cells as described previously (Moffatt et al. 2007). Plasmids were validated by Sanger sequencing using services from the Genome Quebec Innovation Centre.
2.6 Transfection, Immunofluorescence, Binding, and Western Blotting
The plasmids expressing wild type (WT) NPR3 and the two NPR3 variants were tested by transfection into MC3T3-E1 preosteoblastic cells. Cells were seeded in 6-well plates (190,000 cells per well) and grown for 24 h in αMEM (Life Technologies) containing 10% fetal bovine serum (Gibco). The culture media did not contain ascorbic acid, leading to intracellular retention of COL1A1 in the endoplasmic reticulum. One microgram of each plasmid DNA was transfected using XtremeGene9 (Millipore-Sigma) at a 1:6 DNA-reagent ratio. Twenty-four hours later, cells were collected for analysis. For immunofluorescence detection, all steps were performed at room temperature. Cells were washed three times with PBS and fixed for 10 min with 3% (weight/volume) paraformaldehyde. Cells were either left intact (non-permeabilized to reveal only the extracellular cell surface domain) or permeabilized with digitonin (0.01% weight/volume; permeabilizing the plasma membrane but not the membranes of intracellular compartments) for 10 min. After washing with phosphate-buffered saline (PBS), cells were blocked for 1 h at room temperature in PBS containing 2% skim milk and 0.1% bovine serum albumin. Primary antibodies were applied for 2.5 h in the blocking solution at a dilution of 1/1000 (rabbit-anti-FLAG, MilliporeSigma F7425) and 1/300 (mouse-anti-MYC (9E10), Life Technologies 13-2500). For the binding assay, transfected cells were incubated for 2 h with HEK293 conditioned media containing PLAP, PLAP-OSTN, or PLAP-CNP. Cells were washed three times with PBS, fixed, and processed for immunofluorescence staining as described above using a mouse anti-PLAP antibody at 1/300 (Santa Cruz, SC-47691). The secondary goat anti-rabbit (A11012) or anti-mouse (A11005) Alexa Fluor 594-coupled antibodies were applied at 1/1000 (Life Technologies). All cells were counterstained with Hoechst for 5 min (5 μg/mL in PBS; Molecular Probe 33,342) to label the nuclei. Imaging was performed with a 40× PL-Fluotar objective lens by standard epifluorescence on a Leica DMRB microscope equipped with an Olympus DP70 digital camera. All images were captured at the same exposure time. Protein extracts, 8% SDS-PAGE, and western blotting were performed exactly as described previously (Maranda et al. 2022).
To assess the intracellular localization of NPR3, MC3T3-E1 cells were transfected with respective expression plasmids. Twenty-four hours later, cells were processed as described above except that cells were permeabilized for 5 min with 0.1% (v/v) Triton X-100 (which permeabilizes both the plasma membrane and intracellular membranes) in PBS to allow access to the intralumenal compartment of the endoplasmic reticulum. Cells were incubated with a combination of primary anti-FLAG (1/1000) and anti-COL1A1 (EMD AB758 0.4 mg/mL; 1/300) antibodies. Secondary antibodies were applied successively, using first the donkey-antigoat-AF488 (A11055) for COL1A1, and second the goat-anti-rabbit-AF594 (A11012) for FLAG.
3 Results
Two siblings (one female, one male) were initially referred to our institution for orthopedic consultation as they had unusually long halluces that caused discomfort during ambulation. Clinical evaluation in addition revealed tall stature and arachnodactyly. A third sibling did not have these phenotypic features, whereas a fourth sibling, born later, turned out to be similarly affected. The parents were consanguineous and were of Northern African descent. The height of both parents was in the upper part of the reference range (mother: 172 cm, corresponding to a z-score of +1.3; father: 184 cm, corresponding to a z-score of +1.1), resulting in target heights of 184.5 cm (z-score: +1.1) for their male children and 171.5 cm (z-score: +1.3) for their female children. Both parents had a weight above the reference range (mother: 99.5 kg, corresponding to a z-score of +2.8; father: 106.2 kg, corresponding to a z-score of +2.3). Neither of the parents had arachnodactyly or long halluces. There was no family history of cardiac disorders or sudden deaths. The parents did not have a history of significant health issues.
3.1 Individual 1
Individual 1 is a female who was born by spontaneous vaginal delivery at term with a birth weight of 2.9 kg. Birth length was not recorded. She had an umbilical hernia that closed spontaneously. She achieved developmental milestones at the appropriate age (sitting at 8 months; standing at 12 months; walking at 13 months). Cognitive development was normal.
When first evaluated at our institution at 8.3 years of age, Individual 1 was tall (147 cm; z-score +2.8) and weight was 27.7 kg (z-score +0.2), resulting in a BMI of 12.8 kg/m2 (z-score −2.4). Head circumference was 52.5 cm (50th centile). Arm span was 148 cm, arm span-to-height ratio 1.00, and upper-to-lower segment ratio was 0.85. She had arachnodactyly, a positive wrist sign, and a positive thumb sign. The big toes were disproportionately larger and longer than the other toes (Figure 1A). She had elbow hyperlaxity, but finger, knee, and ankle joints were not hypermobile, resulting in a Beighton score of 2 out of 9. There was no skin hyperelasticity. Cardiac auscultation was normal; the abdomen was soft with no hernia detected. Muscle strength appeared normal on physical examination. The postero-anterior spine radiograph revealed scoliosis with a maximal Cobb angle of 30°. There was no sign of malformation of individual vertebrae.
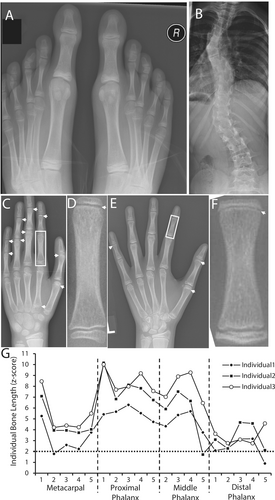
At 9.3 years of age, z-scores for height and weight had slightly increased (Table 1). Lumbar spine areal BMD was below average for age. Forearm pQCT showed normal trabecular and cortical volumetric bone mineral density. Parameters of cross-sectional bone size were within the age- and sex-specific reference range. Muscle cross-sectional area was in the lower half of the reference range. An echocardiogram did not reveal any abnormalities in heart valves or in the diameter of the aortic root. A hand radiograph obtained at 11.3 years of age did not reveal any additional growth plates (not shown).
| Individual 1 | Individual 2 | Individual 3 | |
|---|---|---|---|
| Sex | Female | Male | Male |
| Age (years) | 9.3 | 8.4 | 12.8 |
| Height (z-score) | +2.9 | +4.1 | +4.5 |
| Weight (z-score) | +0.4 | +1.2 | +2.2 |
| Body Mass Index (z-score) | −1.6 | −1.7 | +0.7 |
| Lumbar Spine DXA | |||
| Areal BMD (z-score) | +0.4 | −0.8 | +1.5 |
| Radius pQCT 4% site | |||
| Total cross-sectional area (z-score) | +0.9 | +0.6 | NA |
| Total volumetric BMD (z-score) | −0.4 | +0.04 | NA |
| Trabecular volumetric BMD (z-score) | −0.3 | +0.7 | NA |
| Cortical thickness (z-score) | −0.4 | −1.7 | NA |
| Total bone mineral content (z-score) | +0.8 | +0.9 | NA |
| Radius pQCT 65% site | |||
| Total cross-sectional area (z-score) | −0.1 | −0.3 | NA |
| Cortical cross-sectional area (z-score) | +1.3 | −0.4 | NA |
| Cortical thickness (z-score) | +1.4 | −0.3 | NA |
| Total volumetric BMD (z-score) | +1.3 | −0.1 | NA |
| Cortical volumetric BMD (z-score) | +1.0 | −0.5 | NA |
| Total bone mineral content (z-score) | +1.2 | −0.8 | NA |
| Muscle cross-sectional area (z-score) | −0.7 | −0.8 | NA |
- Note: Results outside of the reference range (z-score below −2 or above +2) are highlighted by bold font.
Despite scoliosis treatment with a brace, the spine curvature progressed in a triple curve pattern with a maximal Cobb angle of 39° (Figure 1B). This was treated with spine fusion surgery at the age of 12 years. To prevent further growth of the hallux, epiphysiodesis of the first metatarsal and the first proximal phalanx of both feet was performed at 12 years of age.
When she was last evaluated at 24 years of age, height was 178.4 cm (z-score +2.4), weight was 68.7 kg (z-score +0.77) and BMI was 21.6 kg/m2 (z-score −0.04). Total body DXA revealed 38.1% body fat (z-score +0.3), Fat Mass/Height2 of 8.21 kg/m2 (z-score −0.2), Lean Mass/Height2 of 12.5 kg/m2 (z-score −1.5) and Appendicular Lean Mass/Height2 of 5.59 kg/m2 (z-score −0.9). Serum P1NP was 40 ng/mL (Norm: 15 to 75 ng/mL); serum CTX was 0.17 ng/mL (Norm 0.05–0.67 ng/mL). Thus, all measured parameters of body composition and bone turnover were within normal limits. A repeat echocardiogram at 24 years of age continued to show normal heart valves and a normal aortic root diameter.
3.2 Individual 2
Individual 2 was born by spontaneous vaginal delivery at term with a birth weight of 3350 g and a birth length of 57 cm. He sat at 7 months and stood independently at 13 months of age. No limb or joint stiffness was noted in early life. His motor and cognitive functions appeared normal.
He was first evaluated at 3.6 years of age. Standing height was 115.7 cm (z-score +3.8), weight was 17.9 kg (z-score: +1.2) and BMI was 13.4 kg/m2 (z-score −2.6). Head circumference was 50 cm (50th centile). The arm span was 118 cm, the arm span-to-height ratio was 1.01, and the upper-to-lower segment ratio was 1.06. He had hypermobile joints with a Beighton score of 7 out of 9 but normal skin elasticity. There was arachnodactyly with a positive thumb sign and a positive wrist sign. The big toes were disproportionately long. He had a high-arched palate but normal teeth. The spine was clinically normal. Chest, cardiac, and abdominal examinations were normal.
Evaluation at the age of 8.4 years showed an increase in height z-score but not in weight z-score, and lumbar spine areal BMD was below average for age (Table 1). Forearm pQCT yielded results at the radius that were within the age- and sex-specific reference range. Muscle cross-sectional area was in the lower half of the reference range. A hand radiograph showed multiple additional growth plates in the phalangeal and metacarpal bones (Figure 1C,D). The lengths of the first metacarpal and proximal phalanx were at a z-scores of +7 and +10, respectively (Figure 1G). At 8.5 years of age, epiphysiodesis was performed bilaterally on the first metatarsals and the first proximal phalanges of both feet. A spine radiograph at 9.1 years of age showed mild curvature with a Cobb angle of 10° and no vertebral malformation. The spine curvature did not progress in subsequent years, and no treatment was required. He was active in sports (basketball) but sustained a left ankle subluxation at the age of 17 years. He also was diagnosed with malocclusion, for which orthodontic treatment was started at 18 years of age. Echocardiograms were performed every other year between 4 and 14 years of age, and in each instance, revealed normal heart valves and normal diameters of the aortic root and of the ascending aorta.
When he was last examined at 19 years of age, height was 211.1 cm (z-score +4.9), weight was 90.8 kg (z-score +1.4) and BMI was kg/m2 (z-score −1.0). The spine radiograph showed mild scoliosis with a Cobb angle of 12°. Lumbar spine areal BMD was (z-score −0.8). Total body DXA could not be performed as his stature exceeded the maximal size for the equipment. Serum P1NP was 137 ng/mL (Norm: 44–225 ng/mL); serum CTX was 0.81 ng/mL (Norm: 0.41–1.70 ng/mL).
3.3 Individual 3
Sibling 3 is a 12-year-old boy with tall stature and a long hallux. He had hyperextensible elbows but no hyperlaxity in fingers or knees, leading to a Beighton score of 2 out of 9. The hand radiograph showed additional growth plates in several phalangeal bones and the first metacarpal (Figure 1E,F). The lengths of the first metacarpal and proximal phalanx were at a z-score of approximately +8 and +10, respectively. The spine radiograph showed mild scoliosis with a Cobb angle of 17° but without signs of vertebral malformation. Total body DXA revealed 33.0% body fat (z-score + 0.9), Fat Mass/Height2 of 6.64 kg/m2 (z-score + 0.7), Lean Mass/Height2 of 12.9 kg/m2 (z-score − 0.8) and Appendicular Lean Mass/Height2 of 6.09 kg/m2 (z-score − 0.4). Serum P1NP was 890 ng/mL (Norm: 450 to 1780 ng/mL), serum CTX was 2.55 ng/mL (Norm: 1.26–3.95 ng/mL). Epiphysiodesis was performed bilaterally on the first metatarsals and the first proximal phalanges of both feet. An echocardiogram performed at 12 years of age showed a structurally normal heart and normal size of the aortic root and the ascending aorta.
3.4 Genetic Testing
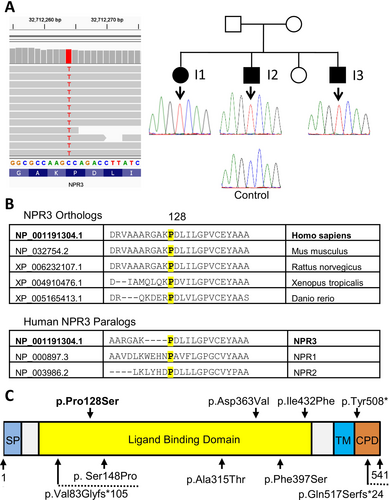
Sequencing of a skeletal disorders gene panel in Individual 3 revealed a homozygous missense variant in NPR3 (NM_001204375.2; c.382C>T; p.Pro128Ser) (Figure 2A). Sanger sequencing confirmed the variant and showed that Individuals 1 and 2 were also homozygous for this variant. Parental samples were not available for testing. The variant is not present in the gnomAD v.4.1.0 database, which contains data on 1,613,364 alleles at this locus (Karczewski et al. 2020). The variant affects an amino acid that is highly conserved in evolution (phyloP100: +7.15) and that is found in all three human NPR proteins (Figure 2B). The amino acid change is predicted to be damaging, with a REVEL score of 0.731, corresponding to an interpretation of ‘pathogenic supporting’ (Pejaver et al. 2022). Five other pathogenic missense variants affecting highly conserved amino acids in NPR3 as well as three variants causing premature termination codons have been described previously in more than four families (Figure 2C) (Boudin et al. 2018; Lauffer et al. 2022). As the variant cosegregated with the disease in all three affected siblings and was associated with a highly specific phenotype, it was classified as likely pathogenic, according to ACMG criteria and relevant guidance documents (Biesecker et al. 2024; Pejaver et al. 2022; Richards et al. 2015).
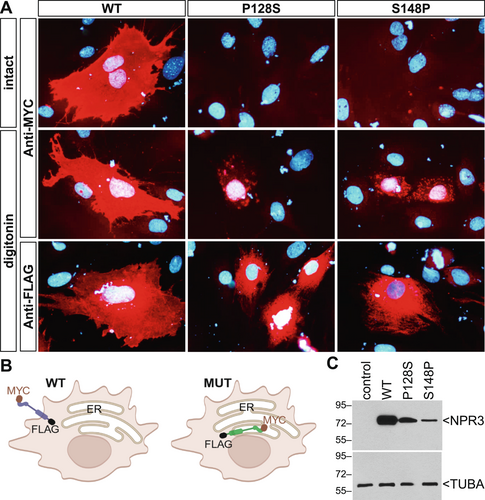
3.5 Functional Experiments
To assess the functional consequences of the NPR3 p.Pro128Ser variant and compare it to the previously described variant p.Ser148Pro, plasmids expressing the wild type NPR3 and the two disease-associated variants were transfected into MC3T3-E1 preosteoblastic cells (Figure 3A). A tagging strategy was employed to better decipher the cellular localization, whereby a MYC epitope was introduced at the N-terminus and a FLAG epitope was introduced at the C-terminus of NPR3 (Figure 3B). Immunofluorescence detection of NPR3 with an anti-MYC antibody on intact (non-permeabilized) cells revealed staining over the plasma membrane only for wild type (WT) NPR3 (Figure 3A). As the MYC tag is expected to be localized in the extracellular N-terminal end of NPR3, the absence of staining for the p.Pro128Ser and p.Ser148Pro NPR3 variants suggested that they do not travel to the plasma membrane. In digitonin permeabilized cells incubated with the anti-MYC, strong labeling was observed over the entire plasma membrane for the WT, while only a few intracellular punctate structures were detectable in cells transfected with either of the two NPR3 variants. WT NPR3 produced an intense and uniform plasma membrane signal with the anti-FLAG when cells were permeabilized with digitonin. In contrast, a distinct and more circumcised reticular pattern was observed with the two NPR3 variants, indicating that they are retained within the endoplasmic reticulum, as depicted in Figure 3B. Endoplasmic reticulum localization of variant NPR3 is demonstrated directly by its co-localization with collagen type I in MC3T3-E1 cells (Figure S1). Western blotting demonstrated that the mutated forms of NPR3 were expressed at slightly lower levels than WT (Figure 3C). NPR3 has a molecular mass of 62 kD, but migrates at an apparently higher molecular weight, as shown by others (Esapa et al. 2016; Matsukawa et al. 1999), presumably because the protein is glycosylated.
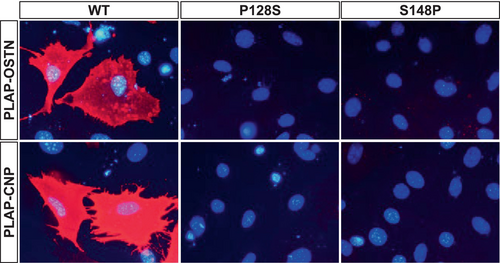
As further proof that NPR3 carrying the p.Pro128Ser and p.Ser148Pro variants is not present at the cell surface, a binding study was performed with two known natural ligands for NPR3, OSTN and CNP. Using PLAP-fusion proteins secreted in the media of HEK293 producing cells, extracellular surface labeling of intact cells was only detected when WT NPR3 was expressed (Figure 4). Cells expressing the p.Pro128Ser and p.Ser148Pro variants were consistently negative.
4 Discussion
In this study, we describe three siblings with a homozygous p.Pro128Ser missense variant in NPR3. The clinical presentation was characterized by tall stature, long halluces, long hand bones, additional growth plates in hands and feet, and scoliosis. Areal bone mineral density at the lumbar spine and volumetric bone mineral densities at the radius were within the age- and sex-specific reference ranges. We found that NPR3 harboring the p.Pro128Ser missense variant was not transported to the cell membrane when it was transfected into MC3T3-E1 preosteoblastic cells.
The phenotype in three siblings reported here is largely similar to the observations that were made in the five previously reported individuals with NPR3-related tall stature (Boudin et al. 2018; Lauffer et al. 2022) (Table 2). Growth velocity seems to be high throughout the postnatal period leading to a steady increase in height z-scores. Apart from tall stature, the clinically most conspicuous finding is the long hallux causing discomfort during ambulation. This was the initial reason for the referral of these individuals to our institution and required surgical intervention with epiphysiodesis in all three siblings. The other unusual finding in NPR3-related tall stature is the presence of additional growth plates in hand and foot bones. A long hallux and the presence of additional growth plates have also been observed in individuals with a heterozygous gain-of-function variant in NPR2, which gives rise to epiphyseal chondrodysplasia Miura type (OMIM 615923) (Lauffer et al. 2020; Miura et al. 2014; Miura et al. 2012). As autosomal dominant epiphyseal chondrodysplasia Miura type and autosomal recessive NPR3-related tall stature are very similar in their clinical manifestations, molecular diagnosis is essential to distinguish between the two disorders and to inform genetic counseling. In addition, following the elucidation of the genetic cause of their disorder, the three siblings underwent cardiac evaluation, as dilatation of the aorta had been described in NPR3-related tall stature (Boudin et al. 2018), but no cardiac or aortic abnormalities were detected.
| Family 1 | Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 5 | Family 5 | |
|---|---|---|---|---|---|---|---|---|
| Individual | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Reported in | Boudin et al. 2018 | Boudin et al. 2018 | Boudin et al. 2018 | Boudin et al. 2018 | Lauffer et al. 2022 | This report | This report | This report |
| Sex | Male | Male | Male | Female | Male | Female | Male | Male |
| Family origin | Dutch | Dutch | Pakistani | NA | Dutch | North Africa | North Africa | North Africa |
| Age (years) at last evaluation | 11 | 10 | 14 | 8 | 14 | 24 | 19 | 12 |
| Height (z-score) | +3.0 | +3.4 | +4.4 | +4.8 | +3.9 | +2.4 | +4.9 | +4.5 |
| Extra epiphyses in fingers and toes | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Long digits (especially halluces) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Aorta dilatation | +3.8 SDS | No | +3 SDS | No | No | No | No | No |
| Joint hypermobility | Yes | Yes | Yes | No | No | No | Yes | No |
| Scoliosis | No | No | No | No | No | Yes | Yes | Yes |
| NPR3 cDNA variant (NM_001204375.2) |
c.442T>C c.1524del |
c.442T>C c.1524del |
c.1088A>T | c.248del |
c.943G>A c.1294A>T |
c.382C>T | c.382C>T | c.382C>T |
| NPR3 protein change |
p.Ser148Pro p.Tyr508* |
p.Ser148Pro p.Tyr508* |
p.Asp363Val | p.Val83Glyfs*105 |
p.Ala315Thr p.Ile432Phe |
p.Pro128Ser | p.Pro128Ser | p.Pro128Ser |
| Zygosity | Compound het | Compound het | Homozygous | Homozygous | Compound het | Homozygous | Homozygous | Homozygous |
A new observation in the present study is that all three siblings had scoliosis. Scoliosis was very mild in the two male siblings, whereas the spine curvature progressed significantly in the female sibling to the point where spinal fusion surgery was eventually needed. The two previous reports on NPR3-related tall stature did not mention a spine phenotype (Boudin et al. 2018; Lauffer et al. 2022), but several individuals with gain-of-function variants in NPR2 had scoliosis (Hannema et al. 2013; Miura et al. 2014; Miura et al. 2012). Interestingly, mouse models with homozygous NPR3 loss-of-function variants do not only have skeletal overgrowth but consistently develop kyphosis (Dauphinee et al. 2013; Esapa et al. 2016; Jaubert et al. 1999). Thus, it seems plausible that scoliosis is a consequence of the NPR3 variant rather than being an unrelated finding in this family. There was no sign of vertebral malformation in any of the individuals presented here, suggesting that they did not have congenital scoliosis but that scoliosis developed over time. Scoliosis is also frequently observed in phenotypically similar conditions such as Marfan syndrome and Miura type epiphyseal chondrodysplasia (Arnaud et al. 2021; Kenis et al. 2021), but the pathomechanisms leading to scoliosis in these conditions are not known in detail.
The two previous reports on humans with NPR3 variants did not include data on markers of bone metabolism and on bone densitometry (Boudin et al. 2018; Lauffer et al. 2022). We found that markers of bone formation and resorption were within normal limits and that both areal BMD at the lumbar spine and volumetric trabecular and cortical BMD at the radius were similar to that of age- and sex-matched reference results. However, given the larger bone size in NPR3-related tall stature, it would be expected that areal BMD is elevated. Similarly, despite tall stature, the cross-sectional size of the radius at the metaphysis and at the diaphysis was only close to average for age and sex in Individuals 1 and 2. This is somewhat surprising in light of data showing that NPR3 is a negative regulator of periosteal bone growth (Watanabe-Takano et al. 2021) and loss of NPR3 function consequently should lead to increased cross-sectional bone size. Our densitometric results therefore suggest that bone mass and size in individuals with loss of NPR3 function are not fully adapted to their increased bone length. Similar observations have been made in individuals with gain-of-function variants in NPR2 (Miura et al. 2014; Miura et al. 2012). Nevertheless, there were no obvious clinical consequences of the relatively low bone mass in our patients, as none of the three siblings had a history of fractures.
In the previously reported individuals with NPR3-related tall stature, the disorder was caused either by missense variants affecting the extracellular domain or by variants leading to premature termination codons (Figure 2). While premature termination codons typically lead to nonsense-mediated decay of mRNA, the mechanism whereby missense variants cause loss of NPR3 protein function is not immediately obvious. In the previously reported NPR3 missense variants, the protein was not transported to the cell membrane (Boudin et al. 2018; Lauffer et al. 2022). In the present study, we confirmed this observation for the previously reported NPR3 p.Ser148Pro variant and in addition found that the same applies to the p.Pro128Ser variant reported here. Interestingly, in three mouse models with different Npr3 missense variants, the disease mechanism involved retention of mutated NPR3 in the endoplasmic reticulum (Esapa et al. 2016). In addition, some common NPR3 missense variants lead to destabilization of the NPR3 protein structure followed by degradation through autophagy (Pereira et al. 2013). The NPR3 p.Pro128Ser and p.Ser148Pro variants may also have led to protein degradation, as Western blots suggested that these missense variants were associated with lower protein levels.
NPR3 is expressed in growth plate cartilage and is thought to act as a decoy receptor for CNP, which is a key regulator of growth plate activity (Rintz et al. 2022). NPR3 thereby decreases the amount of CNP that is available for signaling through its active receptor, NPR2 (Rintz et al. 2022). Biallelic loss-of-function variants in NPR3 therefore increase CNP signaling through NPR2, which results in increased proliferation and differentiation of growth plate chondrocytes and thereby accelerated growth in length.
Even though the clinically most conspicuous findings in individuals with NPR3-related tall stature are related to the skeleton, it is important to note that NPR3 is widely expressed. Loss of NPR3 function may therefore lead to increased CNP signaling in many tissues, which could have clinical consequences. For example, CNP signaling has an antifibrotic effect and decreases collagen production by cardiac fibroblasts (Werner et al. 2023). It is unknown at present whether this causes problems for individuals with NPR3-related tall stature. A genetic analysis based on genome-wide association study data concluded that variants reducing NPR3 function reduce the risk of cardiovascular disease and thus even bring health benefits (Cronje et al. 2023). However, the variants included in that analysis presumably had relatively small effects on NPR3 function, and the conclusion may therefore not be applicable to the loss-of-function variants associated with NPR3-related tall stature. Indeed, aortic dilatation has been observed in some individuals with NPR3-related tall stature (Boudin et al. 2018). Aortic dilatation was not observed in the individuals described here, but it seems prudent to perform echocardiography at regular intervals in individuals with NPR3-related tall stature.
NPR3 is also associated with the regulation of fat mass and obesity, and pharmacologic blockade of NPR3 promotes weight loss (Perez-Ternero et al. 2022; Wang et al. 2023). Low fat mass was noted in some of the previously reported individuals with NPR3 loss-of-function variants as well as in NPR3-deficient mouse models of NPR3 (Boudin et al. 2018; Dauphinee et al. 2013; Esapa et al. 2016; Jaubert et al. 1999). However, we did not observe any abnormality in fat mass or body composition in the two individuals where total body DXA could be performed. Thus, genetic loss of NPR3 function in our patients was not associated with a consistent effect on BMI or body composition.
In conclusion, we found that a novel homozygous loss-of-function missense variant in NPR3 led to the typical features of NPR3-related tall stature and was in addition associated with scoliosis. These observations expand the genotypic and phenotypic spectrum of NPR3-related tall stature.
Author Contributions
Pierre Moffatt: functional molecular analyses, writing and editing. Chantal Janelle: clinical data collection, review and editing. Valancy Miranda: clinical data collection, review and editing. Ghalib Bardai: sequence analysis, review and editing. Frank Rauch: conceptualization, clinical data collection, writing and editing.
Acknowledgments
This work was supported by the Shriners of North America and the Saputo Foundation.
Conflicts of Interest
Pierre Moffatt, Chantal Janelle, Valancy Miranda, and Ghalib Bardai declare no conflicts of interest. Frank Rauch: BioMarin, Ipsen, Kirin, Sanofi, Ultragenyx: consulting fees; Mesentech: study grant to institution.
Open Research
Data Availability Statement
All relevant data are shown in the manuscript. No additional data are available.




