Psychotropic Medication Use in 48,XXYY Syndrome
Funding: This work was supported by the XXYY Project and the eXtraordinarY Kids Clinic at Children's Hospital Colorado, Aurora, CO (NIH/NCATS). The funders assisted with recruiting efforts but had no role in the design and conduct of the study.
ABSTRACT
48,XXYY syndrome is a rare sex chromosome aneuploidy (SCA) condition affecting 1 in 18,000–40,000 male births. Clinical features include tall stature, hypergonadotropic hypogonadism (testosterone deficiency), infertility, developmental delays, learning disabilities, and intellectual impairment. Co-occurring behavior and mental health challenges are common in this population, with high rates of attention-deficit hyperactivity disorder (ADHD), anxiety, depression, sleep disorders, irritability, and aggressive behaviors. We evaluated psychotropic medication use by parent report and retrospective chart review. Treatment success was defined as a positive response per parent report, positive clinician rating, or a treatment duration of at least 6 months. Nearly three-quarters of participants (71/101) with a median age of 15.9 (range 4.5–38 years) had received psychotropic medications. The most common medication classes used were stimulant ADHD medications (78.9% of those with medication use), with a median age of first use of 9 years ([IQR] 7, 11 years), followed by anti-anxiety/antidepressant medications (60.6%), with a median age of first use of 10 years ([IQR] 8, 14 years). Treatment success rates ranged from 43.9% to 84.2% for individual medication trials. Subsequent trials of medications within the same class improved success rates per person in all medication classes except for sleep and mood stabilizer medications. Treatment failure due to side effects was greatest among neuroleptics/atypical antipsychotics, whereas treatment failure due to inefficacy was greatest among anti-anxiety/antidepressants and mood stabilizers. The findings of this study suggest that psychotropic medications targeting behavior and mental health are common and overall helpful for individuals with 48,XXYY.
1 Introduction
48,XXYY syndrome is one of the rarest and most understudied sex chromosome aneuploidy (SCA) conditions, affecting 1 in 18,000–40,000 male births (Blumling et al. 2020; Tartaglia et al. 2008). The syndrome is characterized by wide phenotypic heterogeneity, though almost all individuals demonstrate tall stature, hypotonia, and testosterone deficiency in adolescence due to testicular failure (Fryns et al. 1995; Sorensen et al. 1978; Tartaglia et al. 2011, 2008). There is an increased risk for medical manifestations such as seizures, tremor, Type II diabetes mellitus, and cryptorchidism (Blumling et al. 2020; Tartaglia et al. 2008). In addition to the variety of physical and medical manifestations, individuals with 48,XXYY may experience developmental, neurocognitive, and behavioral challenges secondary to autism, learning disabilities, intellectual disability, and attention-deficit hyperactivity disorder (ADHD) (Blumling et al. 2020; Borja-Santos et al. 2010; Fruhmesser and Kotzot 2011; Sorensen et al. 1978; Srinivasan et al. 2019; Tartaglia et al. 2012, 2017).
Previous literature has described the 48,XXYY phenotype as including attentional difficulties, impulsivity, short frustration tolerance, outbursts, irritability, aggressive behaviors, and anxiety (Blumling et al. 2020; Borghgraef et al. 1991; Borja-Santos et al. 2010; Fryns et al. 1995; Lolak et al. 2005; Srinivasan et al. 2019). Evidence suggests greater psychiatric-related hospitalizations in this patient population. Tartaglia et al. (2008) found that 36.4% of those age > 20 years had a psychiatric-related hospitalization in their lifetime, highlighting the prevalence and severity of mental health and behavioral challenges in this population. However, there is little research specific to psychiatric and neurodevelopmental diagnoses in 48,XXYY. Previous studies report a high prevalence of ADHD, autism spectrum disorder (ASD), anxiety, depression, and mood disorder. One evaluation compared ADHD incidence rates in those with different SCAs and found that of those with XXYY, 72% met DSM-IV criteria for ADHD on parent-report questionnaires compared to 36% in XXY, 52% in XXX, and 76% in XYY. Other evaluations have found that neurodevelopmental and psychological conditions are also common, with ASD in 52%, mood or behavioral disorder in 55%, anxiety in 21%, depression in 18%, obsessive-compulsive disorder in 8%, and bipolar disorder in 8% (Tartaglia et al. 2008, 2012, 2017). There is biological plausibility for this increased risk, as studies have demonstrated that genes on the sex chromosomes are associated with psychiatric conditions (Gao et al. 2015; Piton et al. 2011; Ryan et al. 2015; Zhang et al. 2017).
While clinical guidelines for all neurodevelopmental and behavioral diagnoses generally include therapies paired with medication management when indicated, there are no guidelines to inform management of behavior and mental health challenges in 48,XXYY specifically (Blumling et al. 2020). Clinicians must rely on general guidelines for these patients, even though pathological features are distinct and differences specific to 48,XXYY may lead to variances in responses to pharmacotherapy.
To begin to address this gap, we conducted a study of psychotropic medication use and response to help guide pharmacological management of behavioral and mental health symptoms in individuals with 48,XXYY.
2 Methods
We conducted a cross-sectional study through an online survey and chart review aimed at gathering information on current and past psychotropic medication use. Participants were eligible for inclusion if they had a genetically confirmed diagnosis of 48,XXYY. Participants were recruited from the Medical Investigation of Neurodevelopmental Disorders Institute at University of California Davis Medical Center, the eXtraordinarY Kids Clinic at Children's Hospital Colorado, the Generating Advancements with Longitudinal Analysis in X and Y variations (GALAXY) Registry, and family support groups in the United States, United Kingdom, and Australia. All participants consented to research that was approved by the University of Colorado Investigational Review Board (IRB numbers 08-0513 and 20-0482) or the University of California Davis Investigational Review Board (IRB number 20-04212687). Consent was obtained from the parent or legally authorized representative if < 18. For those ≥ 18 without a legally authorized representative, consent was obtained directly.
The data collection survey regarding psychotropic medication use history was developed in collaboration with the GALAXY Steering Committee. Surveys were completed online via REDCap, a secure, web-based software platform designed to support data capture for research studies (Harris et al. 2009). Surveys were completed between December 2023 and September 2024 in one of three ways: surveys were completed by parents of those with 48,XXYY, by research staff through interviews with parents, or by trained research staff with data abstracted from medical records. Survey instructions encouraged parents to have medical records available when completing the survey. For surveys completed through medical record abstracts, participants were included if they had a documented diagnosis of 48,XXYY syndrome and medication information was available within the health records. Parents were the primary informants due to the sample being primarily pediatric and the high rate of language and cognitive disorders in this genetic condition. For study participants that had reached adulthood, many medications had been started in childhood, and parents were considered the most reliable for recollection of medications and responses. Individuals with XXYY were not excluded from providing survey responses or helping parents complete the survey.
Psychopharmacologic medication survey questions were grouped into the following categories: stimulant ADHD medications, non-stimulant ADHD medications, anti-anxiety/antidepressant medications, sleep medications, neuroleptic/atypical antipsychotics, and mood stabilizers. Specific medications within these groups could be selected. For each medication, respondents indicated age at start of medication, target symptoms, response to target symptom, duration of therapy, reason for stopping medication (if applicable), and medication side effects. The estimated time to complete the survey was 10 min for those with medication use and 30 s for those without.
Surveys completed for participants less than 3 years of age were excluded from analysis due to the unlikelihood of taking psychotropic medications. Total medication trials and the percentage of participants who used a medication were summarized within each class. A medication trial was defined as each distinct course of a medication tried by a participant. For those with multiple medication trials of the same medication, the survey questions were answered for the most recent trial. Rates of positive treatment response were evaluated across medication trials as well as per person. A positive treatment response on a trial level was defined as parent or clinician-reported moderate to significant improvements in at least one target symptom or continuation of the medication for at least 6 months. A positive response at the participant level was defined as a positive response to at least one medication trial within a class.
All summary statistics and data visualizations were generated using R, version 4.4.0. Numeric summaries are presented as median [interquartile range, IQR] due to non-normality, as determined by Shapiro–Wilks. Demographics are presented stratified by history of any medication use, with differences tested using Wilcoxon rank sum tests for continuous variables and Chi-squared tests for categorical variables. Chi-squared tests, or Fisher's exact tests if expected cell counts were < 5, were used to test for a difference in positive response rates between different medications within a class. A reverse Kaplan–Meier curve is used to visualize time, measured as age in years, until first medication use within each class. A Type 1 error rate of 0.05 was assumed.
3 Results
A total of 107 participants completed the survey. Six participants were less than 3 years of age and were excluded from analyses. Thus, 101 total participants were included, with 71 (70.3%) having received at least one psychotropic medication (Table 1). Of the 101 participants, 87 (86.1%) were from the United States while 14 (13.9%) were international. Participants with a history of medication use were significantly older at the time of survey completion compared to participants without a history of medication use (median age 15.9 years [IQR 11.6, 22.5], vs. median age 9 years [IQR 5.4, 14.8; p < 0.001]). Only 5 (15.6%) of the 32 participants over the age of 18 at the time of survey completion had never tried a psychotropic medication. Medication use was more common in United States (64/87; 73.6%) vs. international (7/14; 50%) participants, though not significant (p = 0.112). A total of 273 medication trials occurred across 71 participants (Table 2). Per participant, the highest number of medication trials occurred within the stimulant ADHD medication class, with an average of 1.7 medications per participant, followed by anti-anxiety/antidepressant medications and neuroleptics/atypical antipsychotics, with an average of 1.6 medications per participant.
| All participants | Psychotropic med history (%) | No psychotropic med history (%) | p | |
|---|---|---|---|---|
| N | 101 | 71 (70.3%) | 30 (29.7%) | |
| Age in years (median [IQR]) | 13.9 | 15.9 | 9.0 | < 0.001 |
| [9.4, 20.9] | [11.6, 22.5] | [5.4, 14.8] | ||
| > 18 | 32 (31.9%) | 27 (38%) | 5 (15.6%) | 0.061 |
| Race | 0.168 | |||
| White | 84 (83.2%) | 60 (84.5%) | 24 (80%) | |
| American Indian or Alaska Native | 1 (9.9%) | 1 (1.4%) | 0 | |
| Asian | 1 (9.9%) | 0 | 1 (3.3%) | |
| Mixed race | 2 (19.8%) | 0 | 2 (6.6%) | |
| Other | 4 (4.0%) | 3 (4.2%) | 1 (3.3%) | |
| Unknown | 9 (8.9%) | 7 (9.9%) | 2 (6.6%) | |
| Hispanic/Latinx | 8 (7.9%) | 5 (7%) | 3 (10%) | 0.692 |
| Insurance status a | 0.292 | |||
| Private | 36 (35.6%) | 24 (33.8%) | 12 (40%) | |
| Military | 3 (3%) | 3 (4.2%) | 0 | |
| Medicaid/Medicare | 15 (14.9%) | 10 (14.1%) | 5 (16.7%) | |
| 2 or more | 17 (16.8%) | 15 (21.1%) | 2 (6.7%) | |
| No insurance coverage | 5 (5%) | 4 (5.6%) | 1 (3.3%) | |
| Other | 11 (10.9%) | 5 (7.0%) | 6 (20%) | |
| Unknown | 14 (13.9%) | 10 (14.1%) | 4 (13.3%) | |
| Country | 0.112 | |||
| United States | 87 (86.1%) | 64 (90.1%) | 23 (76.7%) | |
| International b | 14 (13.9%) | 7 (9.9%) | 7 (23.3%) |
- Abbreviations: IQR, interquartile range; Med, medication.
- a Insurance status for international participants was categorized as “other.”
- b Five countries are represented (Canada, Kenya, Australia, UAE, and the United Kingdom).
| Medication | # of participants (%) | # of trials (%) | Age of first trial (years) median [IQR] |
|---|---|---|---|
| Any psychotropic medication | 71 | 273 | 10 [7.75, 12.25] |
| Stimulant ADHD | 56 (78.9%) | 96 (33.3%) | 9 [7, 11] |
| Non-stimulant ADHD | 26 (36.6%) | 33 (11.8%) | 9.5 [8, 12.75] |
| Anti-anxiety/antidepressant | 43 (60.6%) | 62 (22.1%) | 10 [8, 14] |
| Sleep | 18 (25.4%) | 19 (7.3%) | 11 [6, 15.5] |
| Neuroleptic/atypical antipsychotic | 25 (35.2%) | 41 (14.9%) | 11 [8, 12.5] |
| Mood stabilizer | 18 (25.4%) | 22 (8.9%) | 14 [12, 18] |
- Abbreviations: ADHD, attention-deficit hyperactivity disorder; IQR, interquartile range.
Descriptively, the age of first medication trial varied by medication class, with the youngest being for stimulant ADHD medications, followed by non-stimulants and antidepressants/anxiety medications (Table 2, Figure 1).
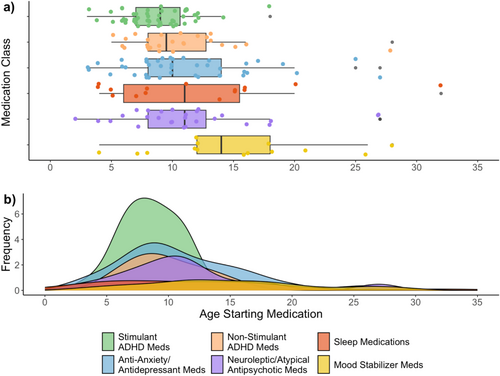
The proportion of all participants having tried each medication class increased with age, with the majority of participants starting medications at school age (age 6–12 years) (Figure 2). By the age of 12, over 50% of participants had trialed a stimulant ADHD medication. The proportion of participants starting an anti-anxiety/antidepressant medication progressively increased from school age into early adulthood (age 6–20 years). At age 12, only about 30% had tried an anti-anxiety/antidepressant, but by age 20, 50% had started a medication in this class. Non-stimulant ADHD medications and neuroleptics/atypical antipsychotics followed a similar pattern, with proportions increasing the most between school age and teenage years (age 8–18 years), but proportions were overall lower than that for stimulant and anti-anxiety/antidepressant medications.
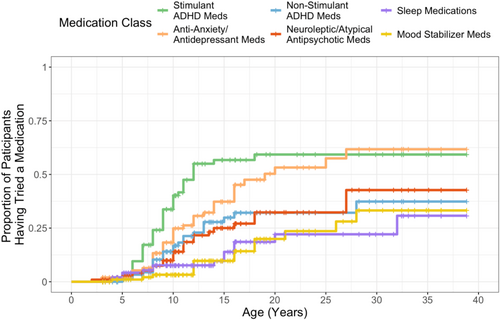
The proportion of positive responses per medication trial ranged from 43.9%–66.7% for stimulants, non-stimulants, anti-anxiety/antidepressants, and neuroleptics/atypical antipsychotic medications (Figure 3). When considering each participant, the proportion of positive responses increased to 64.3%–72% for these classes, indicating additional trials led to increased success. For example, for stimulant medications, there were 96 total trials of stimulants, and 45 of those trials had a positive response. Of 56 participants who tried stimulants, 36 had a positive response to at least one stimulant medication. Positive responses were overall high for sleep medications and mood stabilizers, but decreased slightly at the participant level, indicating that some participants had multiple successful trials, but in those that were not successful, they did not trial multiple medications within the class.
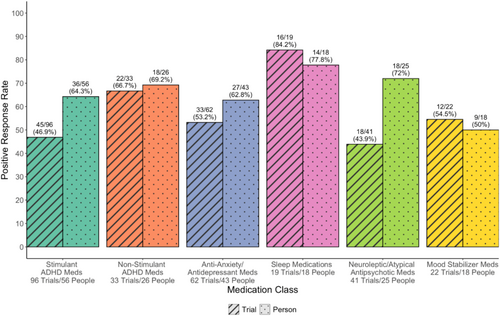
Table 3 provides the breakdown of each medication subclass and individual medications by number of trials and percentages of positive response. Medications were included if at least five medications trials were available for analysis. Among ADHD medications, AMP had significantly higher positive response rate compared to MPH (60.6% vs. 38.1%; p = 0.028) and alpha-2 agonists compared to MPH (80.0% vs. 38.1%; p = 0.008) medications. Within the stimulant and non-stimulant ADHD classes, there was no difference in the proportion of positive responses (p = 0.065 and p = 0.083, respectively). There was additionally no evidence of a difference in positive responses between medications within the anti-anxiety/antidepressant (p = 0.637), neuroleptic/antipsychotic (p = 0.669), or mood stabilizer (p = 0.536) classes. Anti-anxiety/antidepressant medications were relatively successful with 80% and 60% demonstrating positive response for serotonin norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs), respectively. Melatonin was the most common sleep medication, with 86.7% of trials having a positive response.
| Medication | # of trials | Number with positive response | Percent with positive response | p |
|---|---|---|---|---|
| All ADHD medications | 0.013* | |||
| Alpha-2 agonists | 15 | 12 | 80.0 | |
| AMP | 33 | 20 | 60.6 | |
| NRI | 16 | 7 | 43.8 | |
| MPH | 63 | 24 | 38.1 | |
| Stimulant ADHD medications | 0.065* | |||
| Dextroamphetamine and amphetamine | 25 | 16 | 64.0 | |
| Dexmethylphenidate | 13 | 8 | 61.5 | |
| Lisdexamfetamine | 7 | 3 | 42.9 | |
| Methylphenidate | 47 | 16 | 34.0 | |
| Non-stimulant ADHD medications | 0.083* | |||
| Guanfacine | 12 | 10 | 83.3 | |
| Atomoxetine | 16 | 7 | 43.8 | |
| Anti-anxiety/antidepressant medications | 0.637 | |||
| SNRI | 5 | 4 | 80.0 | |
| SSRI | 45 | 27 | 60.0 | |
| Sleep medications | — | |||
| Melatonin | 15 | 13 | 86.7 | |
| Neuroleptic/antipsychotic medications | 0.669* | |||
| Risperidone | 19 | 10 | 52.6 | |
| Aripiprazole | 13 | 5 | 38.5 | |
| Mood stabilizer medications | 0.536* | |||
| Valproic acid | 10 | 7 | 70.0 | |
| Lamotrigine | 7 | 3 | 42.9 | |
- Note: Medications were included if there were at least five trials.
- Abbreviations: AMP, amphetamine stimulants; MPH, methylphenidate stimulants; NRI, norepinephrine reuptake inhibitors; SNRI, serotonin norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors.
- * p-values represent Fisher's exact test for evidence of a difference in positive response rate in at least one medication within a class; significance level = 0.05.
At the time of survey completion, 58 participants were currently taking psychotropic medications. Polypharmacy within psychotropics was low, a majority taking one or two, and only 22.4% taking three or more classes of psychotropic medications at the time of survey completion (Table 4).
| Concurrent psychotropic medication classes per person | Participants (%) |
|---|---|
| 1 | 27 (46.6) |
| 2 | 18 (31.0) |
| 3 | 9 (15.5) |
| 4 | 4 (6.9) |
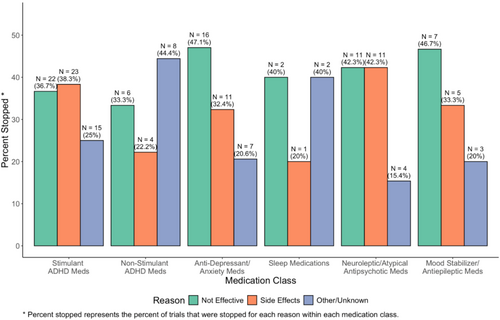
Descriptively, reasons for stopping antidepressant/anxiety medications and mood stabilizers were mostly due to the medication not being effective. Side effects leading to treatment discontinuation were highest among stimulants and neuroleptics/atypical antipsychotics (Figure 4).
4 Discussion
This study presents a descriptive analysis of psychotropic medication use and response in males with 48,XXYY syndrome. From the results presented here, we can conclude that medication use is common, with 70.3% currently or having taken medications for a behavior or mental health indication. ADHD and anxiety/depression were the most common target symptoms. Stimulant ADHD medications were the most commonly used class trialed by over half of the participants, followed by anti-anxiety/antidepressant medications and non-stimulant ADHD medications. This is consistent with high rates of ADHD, anxiety, and depression found in previous literature (Tartaglia et al. 2008). Neuroleptics (also called atypical antipsychotics) are often used for treatment of irritability and/or aggressive behavior, and we found that 35.2% of participants had taken neuroleptic medications, confirming previous reports of these behavior challenges in individuals with 48,XXYY (Blumling et al. 2020; Borghgraef et al. 1991; Borja-Santos et al. 2010; Fryns et al. 1995; Lolak et al. 2005; Srinivasan et al. 2019).
Overall psychotropic medication use of 70.3% in 48,XXYY is higher than the ASD population and similar to Fragile X syndrome, another genetic neurodevelopmental disorder. However, the classes of medications used most appear to be similar across all three conditions. One systematic review of 47 studies and over 300,000 individuals with ASD found prevalence of psychopharmacotherapy ranged from 2.7% to 80% with a median of 45.7% overall, 41.9% in children, and 61.5% in adults (Jobski et al. 2017). Another study evaluating 202 children with ASD ages 5–13 years found 49.5% had used psychotropic medication(s) in the past 6 months, with the most common medication class used being stimulants (58%), followed by anti-anxiety/antidepressants (37%), and non-stimulant ADHD medications (33%) (Caplan et al. 2022). Research on psychotropic medication use in Fragile X found that a similar rate of 72% had been treated with at least one psychopharmacologic agent, with the most common being stimulant ADHD medications (52.9%), followed by anti-anxiety/antidepressants (47.9%) (Berry-Kravis et al. 2012).
It is important to acknowledge differences in psychopharmacologic prescribing practices between different countries, especially in rare disease and pediatric populations. In this study, 14% were from outside of the United States, where it is less common for children to be started on psychotropic medications (Steinhausen 2015). In this sample, there was not a statistical difference but a trend toward the United States being more likely to prescribe medications than other countries. Given the rarity of 48,XXYY syndrome, results can support providers and families outside of the United States in demonstrating utilization and effectiveness of psychopharmacologic medications when providers in those settings may be more hesitant due to the rarity of the condition.
The median age of first medication trial within each class may elucidate the age symptoms are significant enough to require medications. The medication class with the lowest median age at initiation of treatment was the stimulant ADHD medications at 9.0 years of age, which is consistent with high rates of ADHD diagnosis in children with 48,XXYY (Tartaglia et al. 2012). The age of stimulant initiation is similar to that in the general population. One study of 500 electronic health records of children prescribed a stimulant ADHD medication found the mean age of stimulant initiation was 9.3 years (SD = 2.0) (Pasadyn et al. 2020). Use of stimulants earlier than non-stimulant ADHD medications is consistent with the American Academy of Pediatrics ADHD treatment guidelines that recommend stimulant medications as first-line medication therapy (Chatfield and American Academy of Pediatrics 2002). The latest age of first medication trial was for atypical antipsychotics and mood stabilizers at 11 and 14 years, respectively. Onset of use in the adolescent years is consistent with prescribing trends of these medications in ASD and the general population (Canitano 2015; Edelsohn et al. 2017; Park et al. 2016). Later use may also be in part due to reservations of starting these medications because they have a higher likelihood of side effects (Ray et al. 2019; Stroup and Gray 2018). Notably, since this is a primary pediatric sample, decisions to begin and continue treatment are not solely determined by the patient, and parents are the primary decision holders. Additionally, in XXYY more so than in the general population, parents often influence medical care as the patients age into adulthood due to higher rates of intellectual disability (Tartaglia et al. 2008).
Positive treatment response ranged from 43.9% to 84.2% for individual trials. Additional trials led to greater positive response rates (up to 62.8%–72.0%) for stimulants, non-stimulants, anti-anxiety/antidepressants, and neuroleptic/atypical antipsychotics. This demonstrates that these classes of psychotropic medications are overall helpful in most individuals with XXYY, although more than one medication trial within the class may be needed to ultimately achieve success. Clinicians caring for these males with XXYY may consider setting the expectation with families that there is an aspect of trial and error when pursuing psychotropic medication therapy. Additional trials did not necessarily lead to success for sleep and mood stabilizers. Overall, there were lower numbers of trials and participants within these medication classes, which may have influenced results. It is also important to note that our definition of treatment success included those with a moderate to significant improvement in target symptoms or a duration of more than 6 months. We recognize that it is possible a medication may continue for over 6 months if intolerable side effects were not present, even if it was not particularly helpful.
Comparing positive response rates to subclasses of ADHD medications, AMP was greater than MPH (p = 0.028), and alpha-2 agonists were greater than MPH (p = 0.008). Reasons for the significantly higher response rates of AMP compared to MPH are not clear but could in part be due to small differences in their mechanisms of action. While the main mechanism of both agents is to increase synaptic extracellular dopamine and norepinephrine, amphetamine salts also have monoamine oxidase inhibition. Inhibition of monoamine oxidase prevents the breakdown of neurotransmitters dopamine, norepinephrine, and serotonin (Faraone 2018). Clinicians should note that the disparity of positive response rates between AMP and MPH is based largely on participant or parent perception. Guidelines and clinical judgment should still ensue until comparisons are rigorously evaluated in randomized trials.
Positive response rates to MPH and AMP stimulants within our sample are lower than in the general population and in other neurodevelopmental disorder groups. Previous studies examining positive response rates to MPH and AMP in the general pediatric population report rates of 69% (MPH) and 82% (AMP) compared to 38.1% and 60.6% in our population (Greenhill et al. 2001). Positive response rates to MPH within our sample are also lower than that in the ASD population (49%) (Research Units on Pediatric Psychopharmacology Autism Network 2005). Moreover, response rates to MPH are lower than in another genetic neurodevelopmental disorder, Fragile X syndrome, in which a prior study found 56% and 54% positive response rates to MPH and AMP stimulants, respectively (Berry-Kravis et al. 2012). Doses used were not considered in this analysis and could have contributed to lower response rates. For example, if the dose was too low and not titrated up, the medication could have been deemed ineffective. If the dose was initiated too high, side effects could occur and lead to treatment discontinuation. It is also possible that individuals with 48,XXYY experience side effects at doses lower than that required for clinical efficacy.
A majority of participants were currently taking psychotropic medications at the time of survey completion, either on monotherapy or taking two medications for psychotropic indications. While polypharmacy of psychotropics is sometimes necessary, caregivers and medical practitioners involved in treating those with XXYY can be reassured that symptom management can often be accomplished with one or two psychotropic medications.
Stopping medications due to inefficacy was highest among anti-anxiety/antidepressants, neuroleptics/atypical antipsychotics, and mood stabilizers. The high rates of inefficacy in mood stabilizers may be explained by this class being used later in lines of therapy compared to atypical antipsychotics for indications of irritability and aggressive behavior. It may also speak to the refractory nature of patients that trial these medications. Literature investigating the efficacy of mood stabilizers for irritability and mood disorders in the autism patient population has revealed mixed efficacy results which may also hold true in this population as well (Canitano 2015).
Our study had several limitations and important strengths. First, the study design presented many unavoidable biases, including recall bias and negativity bias where negative experiences with medications would be more likely to be recalled. This is especially important in 48,XXYY syndrome where behavioral and medical complications can be quite challenging for families. Next, ascertainment biases existed in that many participants were treated by the authorship team (about 30%) within our specialized interdisciplinary clinic, overrepresenting the prescribing practices of our group. However, our group is also the most experienced in the evaluation and treatment of 48,XXYY syndrome. Additionally, those with more severe symptoms may be more likely to seek care at tertiary care centers and be started on medications. Moreover, chart review and survey responses present limitations in complete data collection. For example, details of all past medication trials may not have been discussed at a clinician visit or may not have been recalled by parents. Therefore, specifics such as the age of medication trial initiation, efficacy, side effects, and reason for stopping medication may not have been documented or known. Also, parents were the primary informants for the survey due to research participants being primarily pediatric and/or having intellectual disabilities. There may have been discrepancies in reported response between parents and the individuals with XXYY that were not accounted for. As with most studies in rare disease conditions, the sample size prevents rigorous analyses, and the lack of statistical significance does not necessarily mean a difference does not exist. However, this is the largest study cohort of 48,XXYY published to date.
Historically, literature regarding those with 48,XXYY syndrome has focused more heavily on physical, medical, or psychological phenotypes with little information available on psychotropic medication use. The findings of this study suggest that psychotropic medications targeting behavior and mental health are common and overall helpful for individuals with 48,XXYY. More rigorous randomized trials evaluating and comparing specific treatment strategies are needed to fully elucidate optimal management, with data collection expanded to include self-reported medication experiences by children and adults with XXYY.
Author Contributions
Dr. Dreyer was responsible for conceptualization, recruiting, methodology, survey writing, data collection, data curation, and writing of the original draft. Ms. Howell was responsible for recruiting participants and review and editing the manuscript. Ms. Bothwell was responsible for data curation, data analysis, statistical analysis, writing the statistics within the methods section, and reviewing and editing the manuscript. Ms. Molison was responsible for recruiting participants, software development, survey writing, and reviewing and editing the manuscript. Ms. Carl was responsible for software development, survey development, and reviewing and editing the manuscript. Dr. Swenson was responsible for data curation, writing the results original draft, and reviewing and editing the manuscript. Dr. Davis oversees the GALAXY consortia that allows participant recruitment and data collection, methodology, survey writing, and reviewing and editing the manuscript. Ms. Gail Decker is responsible for the GALAXY steering committee, recruiting participants, and reviewing and editing the manuscript. The GALAXY consortia was involved in survey design and reviewing and editing the manuscript. Dr. Tartaglia was responsible for conceptualization, funding acquisition, recruiting participants, reviewing and editing the survey, methodology, data analysis, and reviewing and editing the manuscript. All authors critically reviewed the manuscript and agree to be held accountable for all aspects of the work.
Acknowledgments
The authors thank the individuals and caregivers who participated in this study, including those enrolled in the GALAXY Registry, for making this project possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




