Expanding the Phenotypic Spectrum of DPH2-Related Disorder
Funding: The authors received no specific funding for this work.
Vykuntaraju K. Gowda and Varunvenkat M. Srinivasan contributed equally to this study.
ABSTRACT
Biallelic variants in DPH2 have recently been reported to cause the syndrome of developmental delay with short stature, dysmorphic facial features, and sparse hair-2, also known as diphthamide deficiency syndrome-2. Here we report a child with a biallelic loss-of-function variant p.(Arg477*) in DPH2 with clinical features of developmental delay, failure to thrive, sparse hair, seizures that responded to antiepileptics, proportionate short stature, dysmorphism, and hypotonia. Neuroimaging abnormalities were cerebral atrophy, periventricular white matter hyperintensities, and prominent subarachnoid spaces. The electroencephalogram was suggestive of modified hypsarrhythmia. The phenotype of the current case overlaps with the previous cases reported in the literature; however, seizures, behavioral issues, and neuroimaging abnormalities have not been reported to date. This is the third report from the world. The current report gives a detailed account of an Indian child with a DPH2-related disorder.
1 Introduction
Transcription and translation are important biological processes by which a cell regulates gene expression, adapts to various environmental challenges, and maintains cellular homeostasis. The process of translation involves decoding mRNA into proteins. Eukaryotic elongation factor-2 (eEF2), one of the significant components of the translation machinery, functions in the translocation of peptidyl tRNA toward the next codon on mRNA using GTP as an energy source (Hekman et al. 2012; Kaneda et al. 1984).
Further, eEF2 undergoes posttranslational modification with diphthamide, which helps in the modulation of the translational decision at stalled ribosomes and influences levels of various tRNA synthetases. The incorporation of diphthamide into eEF2 involves multiple steps. The first step involves DPH2, encoded by the gene DPH2, which, along with DPH1, forms a heterodimer and produces 3-amino-3-carboxypropyl (ACP) histidine. This intermediate, in further steps, gives rise to diphthamide-modified eEF2. A deficiency of diphthamide in eukaryotic cells leads to changes in cellular oxidative stress response and correlates with early activation of stress pathways. However, the exact function of diphthamide modification is yet to be fully understood (Dong et al. 2014; Liu et al. 2004; Mayer et al. 2019).
Recently, biallelic variants in DPH2 have been reported to cause the syndrome of developmental delay with short stature, dysmorphic facial features, and sparse hair-2 (DEDSSH2: OMIM#620062), also known as diphthamide deficiency syndrome-2 (Hawer et al. 2020). This syndrome presents with delayed developmental milestones, dysmorphic facial features, tone abnormalities, cardiac septal defects, short stature, and hair abnormalities. Only two families are reported to date (Hawer et al. 2020; Makrythanasis et al. 2016). Two other disorders have been described in the diphthamide synthesis pathway with significant phenotype overlap. First, mutations in the DPH1 gene cause diphthamide biosynthesis disorder type-1, also known as Loucks–Innes syndrome (OMIM#616901). Patients clinically manifest with developmental delay, short stature, dysmorphic faces, and sparse hair. The other disorder is a neurodevelopmental disorder with short stature, a prominent forehead, and feeding difficulties (OMIM#620070) caused by mutations in the DPH5 gene (Shankar et al. 2022; Urreizti et al. 2020).
Here, we give a detailed clinical and molecular description of the third family, including additional clinical features, and expand the spectrum of DPH2-related syndromes.
2 Case
The proband is a 1-year 9-month-old female born of a third-degree consanguineous marriage who presented with complaints of delayed attainment of milestones, faltering growth, and seizures. Antenatal ultrasound examinations were normal. The child was delivered at 39 weeks of gestation by cesarean section due to poor progression of labor and cried immediately after birth with no requirement for neonatal intensive care. Weight was 2.5 kg (−1.73 WHO Z), and occipital-frontal circumference (OFC) was 33 cm (−0.74 WHO Z) recorded at birth. The child was feeding well with no evidence of neonatal hyperbilirubinemia or respiratory distress.
Developmentally, neck control was attained at 6 months, rolling by 8 months, sitting with supports, and bi-dextrous reach by 1 year 2 months with no independent sitting, crawling, or standing noted. She demonstrated parental recognition by 3 months and a social smile by 4 months. The child could only speak monosyllables by 1 year 6 months.
The child had multiple episodes of seizures of generalized tonic type and epileptic spasms. The first episode, a generalized tonic type associated with fever, was at the age of 6 months. Four more similar episodes over the next few months were noted, all associated with fever. All were treated with intermittent clobazam prophylaxis. At 1 year 2 months, the child developed another episode of generalized tonic seizures not associated with fever; this time, levetiracetam was started. One month later, the child developed epileptic spasms, initially 2–3 clusters/day with each cluster having 2–4 spasms, and subsequently, the frequency increased to 10–15 clusters/day with each cluster having 8–10 spasms. The child had regression with an increase in spasms; occasional social smiles and no mother recognition, along with irritability and autistic features in the form of repetitive hand movements like clapping and no interaction with surroundings, were noted. At 20 months, the proband visited us and had a good response after starting oral prednisolone and levetiracetam with reduced seizure frequency. The child started gaining milestones after the reduction of spasms. At 1 year 9 months, she could sit with support, an occasional social smile was seen, parental recognition, ambidextrous reach to objects, and stranger anxiety were noted. She attained occasional monosyllables and bisyllables with no meaning. Poor weight gain, feeding difficulty, and constipation were also noted.
On examination, the child had proportionate short stature, sparse hypopigmented hair and eyebrows, broad forehead with high anterior hairline and visible scalp veins, esotropia, depressed nasal bridge, bulbous nose, underdeveloped nasal alae, exaggerated cupid's bow, mandibular prognathia, low-set ears, and brachydactyly in both hands and feet with normal palmar creases (Figure 1A,B). The child had autistic features, which included poor eye contact and repetitive hand movements, along with excessive irritability. Anthropometry showed the following: ofc: 47.5 cm (+0.55 WHO Z), weight: 9.9 kg (−0.75 WHO Z), height: 76 cm (−2.50 WHO Z). Neurological examination showed reduced tone, preserved power, brisk deep tendon reflexes, and upgoing planters. Hair shaft examination under polarized microscopy revealed a pseudo tiger tail appearance (Figure 1C). Electroencephalogram (EEG) at 19 months, on longitudinal montage, showed asymmetric background activity of 2–3 Hz, with multifocal spikes, sharp waves 1–2.5 Hz, and 100–300 μV interictal epileptiform discharges arising from both hemispheres independently, right more than left, suggestive of modified hypsarrhythmia (Figure 1D). Magnetic resonance imaging (MRI) of the brain showed periventricular white matter abnormalities with mild cerebral atrophy and prominent subarachnoid spaces (Figure 1E–H). Metabolic workups, including venous blood gas, vitamin B12, ammonia, lactate, tandem mass spectrometry, and urinary organic acid analysis, were unremarkable. Hearing assessment was normal. Parents are healthy and non-dysmorphic.
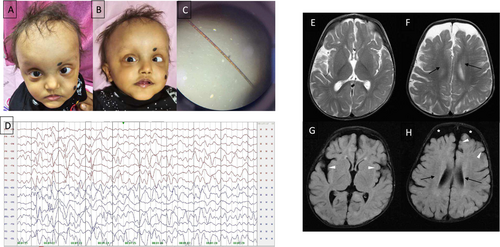
The child is receiving Levetiracetam and Risperidone for seizures and behavioral abnormalities, respectively. Additionally, the child is supported by physiotherapy and speech therapy.
3 Molecular Investigations
3.1 Methods
DNA was extracted from the peripheral blood of the proband. Exome sequencing was performed on the Illumina platform to cover more than 99% of the consensus coding sequence (CCDS) region, with an overall mean depth of 80–100×, with > 90% bases covered at 30× in the target region. Burrows–Wheeler Aligner (Li and Durbin 2009) was used to align the obtained reads on the hg19 reference genome (GRCh37). SNVs and CNVs variant calling was done by the Genome analysis toolkit (GATK) (McKenna et al. 2010). Quality control of reads and exclusion of poor reads were performed on the Sentieon pipeline. Geneyx (version 5.12) was used for variant annotation, analysis, and reporting.
3.2 Results
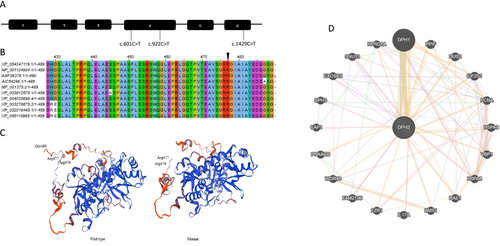
DPH2 was the only candidate gene. The variant identified is a known pathogenic homozygous nonsense variant c.1429C>T, p.(Arg477*), (rs577890255) in Exon-6 of the DPH2 gene. Sanger sequencing in parents showed them to be heterozygous carriers for the identified variant. Mitochondrial genome sequencing was nondiagnostic. The identified nonsense variant is classified as pathogenic as per the American College of Medical Genetics—Association of Molecular Pathology (ACMG-AMP) classification for the interpretation of sequence variants (Richards et al. 2015). The variant is reported in a gene where loss of function is a known mechanism of disease causation (PVS1) and is mapped to the C terminal of the DPH2 protein. The identified variant is seen at extremely low frequency in gnomAD v2.1.1 (https://gnomad.broadinstitute.org/variant/1-44438170-C-T?dataset=gnomad_r2_1) (PM2). The identified variant has been previously reported in ClinVar with two entries (Variation ID: 1687066) as pathogenic/likely pathogenic. The first entry is from OMIM, while the second is an unpublished case of an Indian boy aged between 10 and 19 years with the phenotype of diphthamide deficiency syndrome (https://www.ncbi.nlm.nih.gov/clinvar/variation/1687066/) (PP5). A total of two nonsense and one missense variant has been reported in the DPH2 gene to date (Figure 2A).
4 Conservation Analysis, Insilico Protein Modeling, and Pathway Analysis
4.1 Methods
Protein sequences from BLAST search results were used for multiple sequence alignment (MSA), performed using clustal_X (Larkin et al. 2007). Evolutionary conservation analysis of DPH2 was performed using the ConSurf server (Ashkenazy et al. 2016). The homologs of DPH2 were identified using the protein BLAST. Homology modeling for wild-type and mutant DPH2 was carried out using SWISS-MODEL (Waterhouse et al. 2018) GeneMANIA (Mostafavi et al. 2008) was used for investigating the interactions of DPH2 with other proteins.
4.2 Results
MSA showed that the C-terminal is conserved across organisms, supported by a high conservation score (Figures 2B and S1). Homology modeling suggested a slight change in the structure of mutant DPH2 due to truncation at the C-terminal (Figure 2C). This indicates that the C-terminal may have a key role; thus, truncated DPH2 may affect protein function or stability. DPH2 was found to interact with DPH1 and DPH3 (Figure 2D). DPH2 forms a 2-(3-amino-3-carboxypropyl) histidine synthase complex along with DPH1 and DPH3. This interaction is essential for producing ACP-modified histidine in human eEF2 (Ütkür et al. 2023). It may be the possibility that C-terminal-truncated DPH2 fails to form a complex with DPH1 and DPH3. DPH2 was also found to interact with proteins involved in key cellular processes such as DNA replication (PCNA—proliferating cell nuclear antigen), ribosome biogenesis (WDR46—WD repeat domain 46, BMS1—ribosome biogenesis factor), translation (DUS2—dihydrouridine synthase 2, EIF2S1—eukaryotic translation initiation factor 2), splicing (SNU13—small nuclear ribonucleoprotein 13), mRNA export (RAE1—ribonucleic acid export 1), protein folding (PPIF—peptidylprolyl isomerase F, HSPA8—heat shock protein family A [Hsp70] member 8, HSPA9- heat shock protein family A [Hsp70] member 9), and cell proliferation (FZR1—fizzy and cell division cycle 20 related 1, RPRD1A—regulation of nuclear pre-mRNA domain-containing 1A). The interaction of DPH2 with proteins involved in key cellular processes indicates its essential role in maintaining various cellular processes. This is evident from a broad phenotypic spectrum in patients with DPH2 mutations.
5 Discussion
Here, we report a 21-month-old female with clinical features of delayed developmental milestones, hypotonia, dysmorphic facial features, sparse scalp hair, and short stature. Additional features noted were developmental regression, seizures including epileptic spasms, autistic features, and neuroimaging abnormalities, all of which have not been recorded in the literature concerning this disorder. Only three cases from two families have been reported in the literature (Table 1).
| Number of cases | Makrythanasis et al. (2016) | Hawer et al. (2020) | Current study | |
|---|---|---|---|---|
| Case 1 | Case 2 | 1 | 1 | |
| Genetics | ||||
| DPH2_c.DNA (NM_001384.5) | c.1429C>T | — | c.[922C>T]; [601C>T] | c.1429C>T |
| Protein change | p.(Arg477*) | — | p.[Gln308*]; [Arg201Cys] | p.(Arg477*) |
| Zygosity | Homozygous | — | Compound heterozygous | Homozygous |
| GnomAD (v2.1.1) homozygotes | 0 | — | 0 | 0 |
| Additional genetic variants | KALRN: NM_003947.4: c.3644C>A: p.(Thr1215Lys) in homozygous state | KALRN: NM_003947.4: c.3644C>A: p.(Thr1215Lys) in homozygous state | — | — |
| Clinical features | ||||
| Current age | 15 years | 7 years | 19 months | 21 months |
| Sex | Female | Male | Male | Female |
| Global developmental delay | Yes | Yes | Yes | Yes |
| Seizures | — | — | No | Yes |
| Hypotonia | Yes | Yes | — | Yes |
| Behavioral abnormality | — | — | — | Yes |
| Short stature | Yes | Yes | Yes | Yes |
| Faltering growth | Yes | Yes | — | Yes |
| Microcephaly | Yes | No | No | No |
| Macrocephaly | No | No | Yes | No |
| Dysmorphic features | High forehead, sparse eyebrows, eyelashes, pigmented sclera, prominent nose, low set ears, abnormality of palmar creases | High forehead, sparse eyebrows, eyelashes, pigmented sclera, unilateral ptosis, prominent nose, long philtrum, low set ears, abnormality of palmar creases | High anterior hairline, sparse scalp hair, prominent forehead with visible veins, deep set eyes, posteriorly rotated low set ears, Single palmar crease unilateral, bilateral adducted and infolded thumb. Brachydactyly of all fingers and toes. Soft skin | Sparse hypopigmented hair and eyebrows, broad forehead with high anterior hairline and visible scalp veins, esotropia, depressed nasal bridge, bulbous nose, underdeveloped nasal alae, exaggerated cupid's bow, mandibular prognathia, low-set ears, brachydactyly in both hands and feet |
| Investigations | ||||
| MRI brain | Normal | Normal | Not done, ultrasound brain (6 months): normal | Cerebral atrophy, periventricular hyperintensities and prominent subarachnoid spaces |
| EEG | Normal | Normal | — | Modified hypsarrhythmia |
| Ophthalmological testing | Normal | Normal | — | — |
| Hearing assessment | Normal | Normal | — | Normal |
| Growth hormone deficiency | Yes | Yes | — | — |
| Bone age | Delayed | Delayed | — | — |
| Cardiac abnormalities | — | — | Ventricular septal defect | — |
| Others | Delayed puberty | — | Bilateral hydroceles, notched teeth | Hair shaft examination: pseudo tiger tail appearance |
- Abbreviation: —, information is not available or testing was not done.
The clinical presentations from the previously reported cases were delayed developmental milestones, short stature, tone abnormalities, and dysmorphic facial features, all of which have also been observed in the present case. The common dysmorphic features observed across all four cases include a high anterior hairline, a broad forehead, and sparse scalp hair. The other common features noted were low-set ears, an abnormality of palmar creases, and brachydactyly. There were additional unreported dysmorphic features noted in the current case, which included esotropia, a depressed nasal bridge, a bulbous nose, underdeveloped nasal alae, an exaggerated cupid bow, and mandibular prognathism. The head circumference varied across the cases, with two cases having a normal head size while microcephaly and macrocephaly were noted in one case each. Ventricular septal defect, bilateral hydroceles, and notched teeth were noted in the case reported by Hawer et al. (2020) and were not observed in the present case. Autistic features were noted in the current case, have not been reported previously, and could be part of the neurodevelopmental phenotype.
The current proband developed seizures that included fever-triggered seizures, generalized tonic seizures without fever, and epileptic spasms, which responded to anti-seizure medications. This finding has not been reported in the previous cases. Interestingly, five of the eight patients with DPH1-related disorder, a disorder that occurs in the same pathway as DPH2-related disorder, had seizures. These were described as febrile, tonic, generalized tonic, or absence seizures and ranged in age of onset from the neonatal period up to age 5 years, were easily controlled on medications like what was observed in this case. EEG findings included bilateral occasional hemispheric spikes and generalized slowing, frequent slow runs of generalized spikes, and slow wave discharge (Loucks et al. 2015; Sekiguchi et al. 2018; Urreizti et al. 2020). Similarly, patients with DPH5-related disorder, another disorder that occurs in the same pathway, were reported to have seizures; one of them had myoclonic seizures. EEG abnormalities reported were generalized slowing, multifocal epileptiform discharges, and findings suggesting epileptic myoclonus (Shankar et al. 2022). Seizures are not a consistent feature across all the DPH1 and DPH5 cases, and they have not been reported in patients with DPH2 mutation, which indicates that the seizures might be less penetrant. We will need more cases to know the exact occurrence. The exact mechanism behind the causation of epilepsy is not known and is probably due to a generalized defect in translation machinery.
Phenotypically, patients with mutations in DPH1 and DPH2 have significant overlap. They include developmental delay, short stature, sparse scalp hair, dysmorphism, and limb and tone abnormalities. The dysmorphism involves scalp, forehead, nose, and ear abnormalities. These similarities in phenotype can be attributed to both occurring in a common pathway. They interact strongly, forming a heterodimer that helps form the first intermediate, 3-amino-3-carboxypropyl histidine, an essential step in diphthamide synthesis (Urreizti et al. 2020).
Neuroimaging abnormalities noted in the current case include cerebral atrophy, enlargement of CSF spaces, and T2 periventricular hyperintensities. No neuroimaging abnormalities were detected in siblings reported by Makrythanasis et al., while this was not assessed in the patient reported by Hawer et al. Cerebral atrophy, poorly formed gyri, enlarged ventricles, corpus callosal abnormalities, cerebellar vermis hypoplasia, and Dandy–Walker malformation have been reported in cases with DPH1 mutations (Alazami et al. 2015; Nakajima et al. 2018; Urreizti et al. 2020). The neuroimaging abnormalities reported in DPH5-related disease include cerebral atrophy, white matter abnormalities involving the frontal gyrus and hippocampus, ventricular enlargement, white matter paucity, cerebellar vermian hypoplasia, and minimal subdural hemorrhage. Some patients also had normal neuroimaging (Shankar et al. 2022). The reason for the abnormalities seen on MRI in the current case is likely to be due to the disease pathology and unlikely related to the seizures as they were drug responsive.
The pseudo tiger tail pattern on hair examination appears as alternate dark and light bands spaced irregularly. These are not seen in all hair strands, do not have additional hair abnormalities, and are usually considered nonspecific. It has also been reported in normal individuals (Liang et al. 2005). On the contrary, the tiger tail pattern has regularly spaced alternate light and dark bands with additional trichoschisis and trichorrhexis nodosa-like features, diagnostic of trichothiodystrophy. In the current case, we hypothesize that the pseudo tiger tail is an incidental finding and may not be a diagnostic clue; however, data from additional individuals with DPH2 mutation would give more confidence.
The mutation reported here is a nonsense variant and produces a truncated protein and lies in the C-terminal of the gene. It is unlikely to undergo nonsense-mediated decay, as it lies in the last exon. The region around this variant is conserved as per conservation analysis. InSilico protein modeling demonstrated some changes in the structure compared to the wild type. The current variant needs further studies, including functional assays, to know the exact molecular mechanism for disease causation.
The siblings reported by Makrythanasis et al. had mutations in both KALRN and DPH2 genes. The mutation reported in the siblings and the current case are the same, with significant phenotypic overlap between the two. About the KALRN gene, no OMIM phenotype has been described with this gene to date. Combined association studies have implicated that rare missense variants in KALRN may be a risk factor for Schizophrenia (Kushima et al. 2012). Further, a rare coding missense variant, D1338N in KALRN, was identified in siblings, one with the phenotype of Schizophrenia and the other with major depressive disorder (Russell et al. 2014). The significance of the KALRN mutation on the phenotypic manifestations in the siblings remains unknown. It would be possible that the siblings had a blended phenotype due to the DPH2 and KALRN mutations. However, this would need further studies.
The drawbacks of this study: growth hormone deficiency was not ruled out, and functional studies for the identified variant could not be performed.
To conclude, we report the third case of DPH2-related syndrome and summarize the previously published literature observing a significant phenotype overlap. The novel features noted in this case were the occurrence of seizures, autistic features, neuroimaging abnormalities, and additional dysmorphic features. We recommend including DPH2 in gene panels related to developmental delay and epilepsy. Functional studies on this variant would help elucidate the molecular mechanism behind disease causation.
Author Contributions
V.K.G. and V.M.S. were involved in clinical and genetic evaluation. V.M.S., U.V.K., and P.D. were involved in manuscript writing and data interpretation. S.M.S. was involved in hair microscopy. V.M.S., N.M.P., D.L., and H.P. were involved in interpreting molecular data. V.K.G. approved the final version of the manuscript and stands as a guarantor for it.
Acknowledgments
We thank the patient's family for providing consent for the publication of the data.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




