Heterozygous loss of function variants in IFT140 are associated with polycystic kidney disease
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) affects 1 in 1000 adults. Most cases result from causative PKD1 or PKD2 variants. HNF1B, GANAB and ALG9 variants are also associated with ADPKD. Recent evidence indicates that monoallelic loss-of-function (LoF) IFT140 variants are a cause for non-syndromic ADPKD. We describe 368 patients with IFT140 LoF variants and a spectrum of phenotypic findings that support the association of IFT140 with PKD. We reviewed patients with an unknown cause for their cystic disease and those with heterozygous LoF IFT140 variants classified as pathogenic or likely pathogenic from a cohort that received genetic testing using a panel of 385 renal disease-associated genes. IFT140 LoF variants were significantly enriched in patients with cystic disease when compared with those without cystic disease. A cystic phenotype was reported in 223 of the 368 (60.6%) individuals harboring an IFT140 LoF variant, 98% of which had no other identified cause for their cystic disease. Of 122 unique LoF IFT140 variants identified, 56 (46%) were frameshift, 38 (31%) nonsense, 22 (18%) splice site and 6 (5%) exon-level deletions. Only six IFT140 individuals were reported with end-stage kidney disease, consistent with observed milder clinical presentations in IFT140-related PKD. This study offers further evidence for the involvement of LoF IFT140 variants in PKD, particularly when no additional molecular etiology has been identified.
1 INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic renal disease, affecting 1 in 1000 adults. The diagnosis traditionally carries a 50% risk of progressing to end stage kidney disease (ESKD) by age 60 as well as other non-renal manifestations including vascular and cardiac concerns. Causative variants in PKD1 and PKD2 are identified in the majority of cases with ADPKD, but in a subset of cases, the etiology remains unknown (Mallawaarachchi et al., 2021). Broad panel genetic testing for cystic kidney disease genes has widened the genetic accountability for ADPKD to include genes such as HNF1B, GANAB and ALG9 and has expanded even further into conditions with phenotypic overlap such as autosomal dominant tubulointerstitial disease (ADTKD) and autosomal dominant polycystic liver disease (ADPLD). Clinical diagnosis of ADPKD relies on criteria that take into account cyst number, family history and patient age (Pei et al., 2009). Individuals who do not meet these criteria and present with milder disease or atypical polycystic kidney disease have features that may include a lower number of cysts, unilateral presentation, and normal renal size and function (Lanktree et al., 2019). A causative variant is identified in only 60% of these individuals, much less than those with typical ADPKD, which is reported to be as high as 93% (Cornec-Le Gall et al., 2019; Mallawaarachchi et al., 2021). Genetic diagnosis can benefit these patients by informing selection of potential related kidney donors, assessing risk of disease progression, and guiding clinical management.
Biallelic pathogenic (P) or likely pathogenic (LP) variants in the IFT140 gene are an established cause of non-syndromic retinitis pigmentosa and a spectrum of syndromic findings referred to as short rib thoracic dysplasia where multisystem involvement varies in severity, but is widespread across retinal, cerebral, renal and skeletal tissues. More specifically, renal presentations in these patients include chronic kidney disease, cysts and interstitial fibrosis (Schmidts et al., 2013). Senum et al. (2022) was the first to show that monoallelic loss-of-function (LoF) IFT140 variants, which affect the function of the IFT140 protein, a component of the IFT-A complex that is involved in signaling and protein trafficking out of cilia (Picariello et al., 2019) are a cause of a non-syndromic ADPKD phenotype. They further described a cystic phenotype that contrasts with the typical severity associated with PKD1- and PKD2-related ADPKD. Additionally, Chang et al. (2022) provided further support of an IFT140-associated cystic phenotype. Here, we describe the genetic and phenotypic findings of a cohort of patients with monoallelic LoF IFT140 variants that were gleaned from the analysis of 41,182 consecutive individuals for whom genetic testing was performed with a 385 kidney gene panel designed for patients with CKD. This study provides additional evidence for loss of function variation in IFT140 as a cause for a mild form of PKD.
2 METHODS
Genetic testing and variant interpretation were performed as described in Bleyer et al. (2022). Briefly, individuals who received genetic testing with a 385 kidney disease gene panel (the RenasightTM test, Natera Inc., Austin, Texas) between May 2020 and December 2022 were reviewed.
Variants were assessed based on the American College of Medical Genetics and Genomics and Association for Molecular Pathology (ACMG/AMP) guidelines for sequence variant interpretation (Richards et al., 2015). For dominant or X-linked conditions, a heterozygous P or LP variant in a gene was reported as a positive result (diagnostic).
Given the established autosomal recessive conditions associated with the IFT140 gene (short-rib thoracic dysplasia 9 with or without polydactyly/retinitis pigmentosa 80), heterozygous IFT140 LoF variants (predicted loss of function variants for the purpose of this study excluded missense variants; NCBI GenBank number NM_014714.4) were reported as carrier variants (non-diagnostic). These individuals were identified for this study.
Demographic information (e.g., age, sex, and ethnicity) and clinical information were provided by the physician based on patient (or guardian, in the case of minors) report. ICD-10 codes and/or clinical information provided by the clinician were used to categorize patients as affected. For this study, when physicians provided ICD-10 codes that indicated polycystic kidney disease (Q61.0-.9), acquired kidney cysts (N28.1) and chronic kidney disease (N18.1-6), phenotypic analysis was performed.
Known genetic causes of autosomal dominant cystic disease were considered by the presence of a heterozygous P or LP variant in PKD1, PKD2, ALG9, HNF1B, GANAB, UMOD, SEC63, and/or PRKCSH. An initial exploratory analysis was performed to identify genes that were enriched in individuals with reported cystic phenotypes that had no attributable genetic cause of disease. This was achevied by comparing the prevalence of variants in the other panel genes in the cohort of individuals without a P/LP variant in the genes listed above to those with a P/LP in one or more of those genes. The significance of the enrichment of a given gene within one population was assessed using Fisher's exact test with Bonferroni adjustments for multiple comparisons. In a subsequent IFT140-specific analysis, odds ratios were calculated between individuals with and without reported cystic phenotypes; significance of the odds ratios were assessed using Fisher's exact test. Data regarding prevalence of alleles in a healthy population was obtained from gnomAD V.2.1.1 (Karczewski et al., 2020).
All patients or legal guardians (in cases of minors) provided informed consent for the performance of genetic testing. Data were de-identified prior to analysis. The study was performed in adherence with the Declaration of Helsinki. An exemption from institutional review board review (study ID 20099-03) was received from Ethical & Independent Review Services, Corte Madera, California.
3 RESULTS
3.1 IFT140 was identified as a top candidate as a cause of cystic disease in individuals with no known genetic cause
An unbiased, retrospective analysis of 3789 individuals with an ICD-10 code indicative of cystic disease (Q61.0-.9 and/or N28.1) was performed to identify genes that were enriched among individuals without an attributable genetic cause for their disease. Within the group of individuals with a reported cystic disease, 37.6% (n = 1425) had predicted P or LP variants in at least one known cystic-related gene (PKD1, PKD2, ALG9, HNF1B, GANAB, UMOD, SEC63, PRKCSH). The remaining 62.4% (2364) of individuals did not have a causative variant in a cystic-related gene. Comparison of individuals with no known causal variant in a cystic-related gene and those with a known cystic disease cause revealed a significant enrichment in heterozygous variants in the IFT140 gene (8.5% [200/2364] vs. 3.4% [49/1425]; p < 0.001, Bonferroni corrected p-value < 0.001).
3.2 Individuals with IFT140 LoF variants are at higher odds of having cystic disease
Given the enrichment of heterozygous IFT140 variants in a cohort of individuals with no known cause of cystic disease, and the reported association of monoallelic IFT140 LoF variants with ADPKD (Senum et al., 2022), we performed a subsequent query of LoF IFT140 variants within a database of 41,182 ethnically and racially diverse individuals who received a broad panel renal genetic test result between May 2020 and December 2022 (Table S1). Among 368 individuals identified with a heterozygous IFT140 LoF variant irrespective of a cystic phenotype, 97% (n = 357) individuals did not have an identified genetic cause of cystic disease (genes listed above). Within this cohort, the odds of harboring a heterozygous IFT140 LoF variant in the absence of a P/LP in a known cystic gene were significantly higher among patients with cystic disease (n = 219 with IFT140 LoF variant vs. n = 2476 without IFT140 LoF variant) than those without cystic disease (n = 138 with IFT140 LoF variant vs. n = 34,993 without IFT140 LoF variant) (OR: 22.42 [95% CI: 17.9575–28.0810]; p-value <0.0001).
3.3 The IFT140 LoF variant landscape
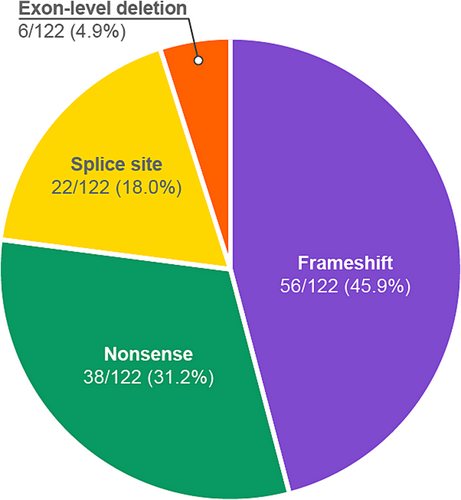
Of the 368 individuals identified with heterozygous predicted LoF P or LP variants in the IFT140 gene, 171 [46.5%] were female and 197 [53.5%] were male, ranging in age from 4 months to 90 years (Figure 1). Among the 122 unique IFT140 variants with a predicted LoF effect, 56 (46%) were frameshift, 38 (31%) nonsense, 22 (18%) splice site and 6 (5%) exonic deletions (Figure 2). Of these 122 variants, 116 (95%) were represented in the gnomAD database in 4 or fewer alleles, of which, 78 (64%) were absent entirely from the database (Table 1) and 66 (54.0%) had not been reported before in literature (Table S2).
| Variant | Exon | Number of cases (% of cohort) | MAF in affected cohort (n = 41,182) | Prevalence in gnomAD (V2.1.1) |
|---|---|---|---|---|
| c.2399 + 1G > T (p.?) | 19 | 61 (16.60%) | 0.148% | <0.01% (14/251478 alleles) |
| c.1377G > A (p.Trp459*) | 12 | 58 (15.80%) | 0.141% | <0.01% (22/251228 alleles) |
| c.1959G > A (p.Trp653*) | 17 | 16 (4.30%) | 0.039% | <0.01% (4/280738 alleles) |
| c.2767_2768 + 2del (p.Tyr923Leufs*28) | 21 | 16 (4.30%) | 0.039% | <0.01% (8/148386 alleles) |
| c.1010-1G > A (p.?) | 10 | 11 (3.00%) | 0.027% | <0.01% (11/279352 alleles) |
| c.2214_2217del (p.Asp738Glufs*47) | 19 | 9 (2.40%) | 0.022% | absent |
| c.2500C > T (p.Arg834*) | 20 | 6 (1.60%) | 0.015% | <0.01% (2/256536 alleles) |
| c.1648C > T (p.Arg550*) | 14 | 6 (1.60%) | 0.015% | absent |
| c.1513C > T (p.Arg505*) | 13 | 5 (1.40%) | 0.012% | <0.01% (5/251362 alleles) |
| c.1009 + 1G > T (p.?) | 10 | 5 (1.40%) | 0.012% | <0.01% (1/31410 alleles) |
| c.1147C > T (p.Gln383*) | 10 | 5 (1.40%) | 0.012% | <0.01% (1/31360 alleles) |
| c.889G > T (p.Glu297*) | 8 | 4 (1.10%) | 0.010% | absent |
| c.1525-1G > A (p.?) | 14 | 4 (1.10%) | 0.010% | absent |
| c.1901 + 1G > T (p.?) | 16 | 4 (1.10%) | 0.010% | <0.01% (4/250370 alleles) |
| c.2598C > G (p.Tyr866*) | 21 | 3 (0.80%) | 0.007% | <0.01% (3/213880 alleles) |
| c.2638C > T (p.Gln880*) | 21 | 3 (0.80%) | 0.007% | absent |
| c.2872_2875delinsGT (p.Trp958Valfs*161) | 23 | 3 (0.80%) | 0.007% | absent |
| c.2880G > A (p.Trp960*) | 23 | 3 (0.80%) | 0.007% | absent |
| c.3219C > A (p.Tyr1073*) | 25 | 3 (0.80%) | 0.007% | <0.01% (1/250086 alleles) |
| c.565del (p.Ser189Alafs*7) | 6 | 3 (0.80%) | 0.007% | absent |
| c.2948del (p.Asp983Alafs*21) | 23 | 3 (0.80%) | 0.007% | absent |
| c.151G > T (p.Glu51*) | 4 | 3 (0.80%) | 0.007% | <0.01% (1/31394 alleles) |
| c.1867_1870del (p.Glu623Argfs*20) | 16 | 3 (0.80%) | 0.007% | <0.01% (2/282858 alleles) |
| c.1909del (p.Leu637Serfs*7) | 17 | 3 (0.80%) | 0.007% | <0.01% (1/238946 alleles) |
| c.3521del (p.Met1174Argfs*22) | 27 | 3 (0.80%) | 0.007% | <0.01% (1/248928 alleles) |
| c.3874-1G > A (p.?) | 29 | 3 (0.80%) | 0.007% | <0.01% (3/276940 alleles) |
| c.1406_1430del (p.Leu469Glnfs*11) | 12 | 2 (0.50%) | 0.005% | absent |
| c.1536_1539del (p.Lys512Asnfs*24) | 14 | 2 (0.50%) | 0.005% | absent |
| c.1644_1645del (p.Ser549Profs*9) | 14 | 2 (0.50%) | 0.005% | absent |
| c.2411G > A (p.Trp804*) | 20 | 2 (0.50%) | 0.005% | <0.01% (1/238800 alleles) |
| c.2563C > T (p.Gln855*) | 20 | 2 (0.50%) | 0.005% | absent |
| c.2944_2948delinsGGGG (p.Arg982Glyfs*22) | 23 | 2 (0.50%) | 0.005% | absent |
| c.332G > A (p.Trp111*) | 4 | 2 (0.50%) | 0.005% | absent |
| c.3331C > T (p.Gln1111*) | 26 | 2 (0.50%) | 0.005% | <0.01% (3/251034 alleles) |
| c.3378_3384delinsCCGACTTCTTCGGAGCACAGTCAGTG (p.Ala1127Argfs*65) | 26 | 2 (0.50%) | 0.005% | absent |
| c.3939C > A (p.Cys1313*) | 29 | 2 (0.50%) | 0.005% | <0.01% (3/275566 alleles) |
| c.611del (p.Leu204Cysfs*6) | 6 | 2 (0.50%) | 0.005% | absent |
| Deletion of Exon 14 | – | 2 (0.50%) | 0.005% | absent |
| Deletion of Exons 20–21 | – | 2 (0.50%) | 0.005% | absent |
| Deletion of Exons 8–14 | – | 2 (0.50%) | 0.005% | absent |
| c.3916dup (p.Ala1306Glyfs*56) | 29 | 2 (0.50%) | 0.005% | <0.01% (4/272218) |
| c.2278C > T (p.Arg760*) | 19 | 2 (0.50%) | 0.005% | absent |
| c.1422_1423insAA (p.Arg475Asnfs*14) | 12 | 2 (0.50%) | 0.005% | absent |
| c.1039C > T (p.Arg347*) | 10 | 2 (0.50%) | 0.005% | <0.01% (1/250764 alleles) |
| c.1072del (p.Leu358Trpfs*38) | 10 | 2 (0.50%) | 0.005% | <0.01% (1/251276 alleles) |
| c.1525-2_1525-1del (p.?) | 14 | 2 (0.50%) | 0.005% | <0.01% (1/246836 alleles) |
| c.240G > A (p.Trp80*) | 4 | 2 (0.50%) | 0.005% | <0.01% (1/31392 alleles |
| c.299del (p.Leu100Argfs*45) | 4 | 2 (0.50%) | 0.005% | <0.01% (1/251264 alleles) |
| c.3250_3253dup (p.Val1085Glyfs*36) | 7 | 2 (0.50%) | 0.005% | <0.01% (1/249572 alleles) |
| c.3408_3409del (p.His1136Glnfs*49) | 26 | 2 (0.50%) | 0.005% | <0.01% (2/250382 alleles) |
| c.409C > T (p.Arg137*) | 5 | 2 (0.50%) | 0.005% | <0.01% (2/251240 alleles) |
| c.581del (p.Leu194Cysfs*2) | 6 | 2 (0.50%) | 0.005% | absent |
| c.1073_1083del (p.Leu358Argfs*122) | 10 | 1 (0.30%) | 0.002% | absent |
| c.1158G > A (p.Trp386*) | 11 | 1 (0.30%) | 0.002% | absent |
| c.1182del (p.Val395*) | 11 | 1 (0.30%) | 0.002% | absent |
| c.1359_1359 + 3delinsAC (p.Asp454Argfs*30) | 11 | 1 (0.30%) | 0.002% | absent |
| c.1360-2A > G (p.?) | 12 | 1 (0.30%) | 0.002% | absent |
| c.146_147 + 2del (p.Gln49Argfs*40) | 3 | 1 (0.30%) | 0.002% | absent |
| c.1522C > T (p.Gln508*) | 13 | 1 (0.30%) | 0.002% | absent |
| c.157_160del (p.Val53Glnfs*32 | 4 | 1 (0.30%) | 0.002% | absent |
| c.1814_1824dup (p.Val609Lysfs*5) | 16 | 1 (0.30%) | 0.002% | absent |
| c.1823_1824del (p.Thr608Serfs*5) | 16 | 1 (0.30%) | 0.002% | absent |
| c.1963C > T (p.Gln655*) | 17 | 1 (0.30%) | 0.002% | absent |
| c.217_218del (p.Arg73Alafs*16) | 4 | 1 (0.30%) | 0.002% | absent |
| c.2200-1_2200delinsAG (p.?) | 19 | 1 (0.30%) | 0.002% | absent |
| c.2200-1G > A (p.?) | 19 | 1 (0.30%) | 0.002% | absent |
| c.2223_2224dup (p.Glu742Alafs*45) | 19 | 1 (0.30%) | 0.002% | absent |
| c.2286del (p.Phe762Leufs*24) | 19 | 1 (0.30%) | 0.002% | absent |
| c.2292del (p.Leu765Trpfs*21) | 19 | 1 (0.30%) | 0.002% | absent |
| c.2317del (p.Arg773Glyfs*13) | 19 | 1 (0.30%) | 0.002% | absent |
| c.2443C > T (p.Gln815*) | 20 | 1 (0.30%) | 0.002% | absent |
| c.2464del (p.Val822Cysfs*24) | 20 | 1 (0.30%) | 0.002% | <0.01% (2/238810 alleles) |
| c.2539del (p.Ala847Profs*29) | 20 | 1 (0.30%) | 0.002% | absent |
| c.2544_2547dup (p.Ala850Argfs*103) | 20 | 1 (0.30%) | 0.002% | absent |
| c.2578-2A > G (p.?) | 21 | 1 (0.30%) | 0.002% | absent |
| c.2671del (p.Val891*) | 21 | 1 (0.30%) | 0.002% | absent |
| c.2677G > T (p.Glu893*) | 21 | 1 (0.30%) | 0.002% | <0.01% (1/213236 alleles) |
| c.2682delinsAA (p.His894Glnfs*58) | 21 | 1 (0.30%) | 0.002% | absent |
| c.2768 + 1dup (p.?) | 21 | 1 (0.30%) | 0.002% | absent |
| c.2768 + 1G > A (p.?) | 21 | 1 (0.30%) | 0.002% | absent |
| c.2769-1G > T (p.?) | 22 | 1 (0.30%) | 0.002% | absent |
| c.2844C > G (p.Tyr948*) | 22 | 1 (0.30%) | 0.002% | absent |
| c.2882G > A (p.Trp961*) | 23 | 1 (0.30%) | 0.002% | absent |
| c.2947dup (p.Asp983Glyfs*137) | 23 | 1 (0.30%) | 0.002% | absent |
| c.2980C > T (p.Gln994*) | 23 | 1 (0.30%) | 0.002% | absent |
| c.2997 + 1G > C (p.?) | 23 | 1 (0.30%) | 0.002% | absent |
| c.2998-4_2998-2delinsTT (p.?) | 24 | 1 (0.30%) | 0.002% | absent |
| c.301del (p.Thr101Hisfs*44) | 4 | 1 (0.30%) | 0.002% | absent |
| c.3070G > T (p.Glu1024*) | 24 | 1 (0.30%) | 0.002% | absent |
| c.308delinsAG (p.Thr103Lysfs*75) | 4 | 1 (0.30%) | 0.002% | absent |
| c.3160C > T (p.Gln1054*) | 25 | 1 (0.30%) | 0.002% | absent |
| c.3270 + 2 T > G (p.?) | 26 | 1 (0.30%) | 0.002% | absent |
| c.3361_3362del (p.Ser1121Argfs*64) | 26 | 1 (0.30%) | 0.002% | absent |
| c.3493del (p.Ser1165Alafs*10) | 27 | 1 (0.30%) | 0.002% | absent |
| c.3617del (p.His1206Profs*14) | 27 | 1 (0.30%) | 0.002% | absent |
| c.3763C > T (p.Gln1255*) | 28 | 1 (0.30%) | 0.002% | absent |
| c.3962del (p.Ser1321Thrfs*19) | 29 | 1 (0.30%) | 0.002% | absent |
| c.39dup (p.Ala14Cysfs*76) | 3 | 1 (0.30%) | 0.002% | absent |
| c.54dup (p.Ser19Leufs*71) | 3 | 1 (0.30%) | 0.002% | absent |
| c.854del (p.Leu285*) | 8 | 1 (0.30%) | 0.002% | absent |
| c.872_900del (p.Leu291Glnfs*17) | 8 | 1 (0.30%) | 0.002% | absent |
| c.996C > G (p.Tyr332*) | 9 | 1 (0.30%) | 0.002% | absent |
| Deletion of Exon 4–18 | – | 1 (0.30%) | 0.002% | absent |
| Deletion of Exons 20–23 | – | 1 (0.30%) | 0.002% | absent |
| Deletion of Exons 20–25 | – | 1 (0.30%) | 0.002% | absent |
| c.1380del (p.Asn460Lysfs*28) | 12 | 1 (0.30%) | 0.002% | <0.01% (1/251220 alleles |
| c.1655_1656del (p.Glu552Glyfs*6) | 15 | 1 (0.30%) | 0.002% | absent |
| c.2712C > G (p.Tyr904*) | 21 | 1 (0.30%) | 0.002% | absent |
| c.1155 + 1G > A (p.?) | 10 | 1 (0.30%) | 0.002% | <0.01% (1/249872 alleles |
| c.1433-1G > A (p.?) | 13 | 1 (0.30%) | 0.002% | <0.01% (1/251104 alleles) |
| c.1501C > T (p.Arg501*) | 13 | 1 (0.30%) | 0.002% | <0.01% (1/251388 alleles) |
| c.1712dup (p.Ile572Hisfs*21) | 15 | 1 (0.30%) | 0.002% | <0.01% (1/31374 alleles) |
| c.200_212dup (p.Thr72Valfs*22 | 4 | 1 (0.30%) | 0.002% | <0.01% (3/251288 alleles) |
| c.2068-2A > G (p.?) | 18 | 1 (0.30%) | 0.002% | <0.01% (1/220192 alleles) |
| c.2655del (p.Trp885Cysfs*7) | 21 | 1 (0.30%) | 0.002% | <0.01% (1/225042 alleles) |
| c.2987del (p.Asn996Metfs*8) | 23 | 1 (0.30%) | 0.002% | <0.01% (1/250184 alleles) |
| c.308_309del (p.Thr103Serfs*74) | 4 | 1 (0.30%) | 0.002% | <0.01% (1/251266 alleles) |
| c.634 + 5G > A (p.?) | 6 | 1 (0.30%) | 0.002% | <0.01% (5/282706 alleles) |
| c.3214C > T (p.Arg1072*) | 25 | 1 (0.30%) | 0.002% | <0.01% (3/249956 alleles) |
| c.1359 + 1G > A (p.?) | 11 | 1 (0.30%) | 0.002% | absent |
| c.223del (p.Val75Cysfs*11) | 4 | 1 (0.30%) | 0.002% | absent |
| c.2483del (p.Gly828Alafs*18) | 20 | 1 (0.30%) | 0.002% | <0.01% (1/235928 alleles) |
In our cohort, 57.4% (n = 70) of the variants were private (i.e. unique to an individual/family). The minor allele frequencies (MAFs) of all reported variants from this cohort were higher than those reported in the gnomAD database, as would be expected for a primarily affected cohort (Table 1). The c.2399 + 1G > T (p.?) variant that was identified in 61 individuals (16.6%), was the most common variant in this cohort, followed by, c.1377G > A (p.Trp459*), a nonsense variant that was observed in 58 individuals (15.8%) and c.1959G > A (p.Trp653*), that was observed in 16 individuals (4.3%). Exonic deletions, which were predicted to result in a frameshift or the loss of a significant portion of the coding region of the gene, ranged from single exons to deletions encompassing 15 of the 29 coding exons. Of all 29 coding exons in IFT140 (NM_014714.4 exons 3–31), variants were identified in all exons except 30 and 31, which is expected based on predicted LoF variants escaping nonsense-mediated decay in the last exon or the last 50 bp of the penultimate exon.
3.4 Disease phenotypes in patients with IFT140 LoF variants
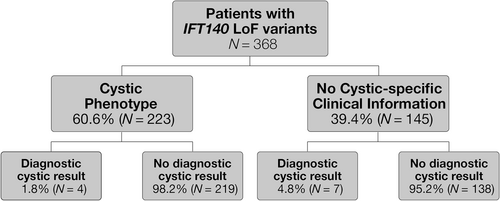
Among the 368 individuals with LoF variants in IFT140, 60.6% (n = 223) had clinical histories that indicated a cystic phenotype. Four (1.8%) individuals with a reported cystic phenotype had a positive finding in a known cystic disease gene (HNF1B [n = 1], PKD1 [n = 2], IFT140 AR disease [n = 1, LoF/missense compound heterozygote]), leaving 98% (219/223) without an otherwise identified cause for their cystic disease. Of the 145 individuals with an IFT140 LoF variant for which a cystic phenotype was not reported, 91 (62.8%) were only referred with stage of kidney disease and no phenotypic descriptors, whereas the remaining 54 (37.2%) reported various non-cystic renal phenotypes. Seven (4.8%) of these individuals had a positive finding in a known cystic disease gene (ALG9 [n = 1], PKD1 [n = 3], UMOD [n = 2], IFT140 AR disease [n = 1, LoF/missense compound heterozygote]). Altogether, 97.0% (357/368) of those with an IFT140 LoF variant had no other diagnostic result for cystic disease (Figure 1). It is important to note that since the presence of cystic disease was based on ICD-10 code reporting, these numbers may be underestimated.
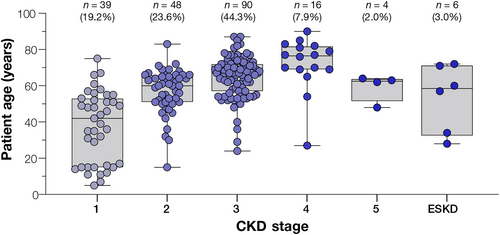
ADPKD associated with IFT140 LoF variants is proposed to be of later onset and milder in severity than classic PKD1 and PKD2- associated disease (Senum et al., 2022). At the time of testing, CKD stage was available for 56.9% (203/357) individuals in our cohort with an IFT140 LoF variant and no other diagnostic result for cystic disease (regardless of whether cystic phenotype was indicated or not). Of the 203 individuals, Stage 3 was the most commonly reported stage (44.3%), whereas CKD stage 5/ESKD were rarely reported (4.9%, combined; including one patient with a kidney transplant) (Figure 3). The majority (82.5%; [94/114]) of patients aged 60+ had CKD Stage 3 or lower, and only three patients in this age group had progressed to ESKD. There were 31 individuals (8.7%) with an IFT140 LoF variant who were under 18 years of age (range: 4 months to 17 years) at the time of testing, of which, the stage of disease was provided for 13 individuals. Aside from one 15 year old with stage 2 disease, the remaining 12 (92.3%) were reported as CKD stage 1. Cystic disease was provided as the indication for genetic testing for 15 (48.4%) individuals younger than 18, none of whom had a positive result in another cystic disease gene.
4 DISCUSSION
Reports have indicated that causative variants are identified in only 60% of individuals with atypical presentations of ADPKD (i.e. do not meet diagnostic clinical criteria) (Mallawaarachchi et al., 2021). Recent reports indicate mono-allelic loss-of-function (LoF) variants in the IFT140 gene cause atypical ADPKD that is milder than the phenotype classically associated with PKD1 and PKD2 and that they comprised the third most common gene association with cystic kidney disease behind PKD1 and PKD2. These variants are also likely associated with a later onset of renal insufficiency that infrequently reaches ESKD (Senum et al., 2022). Evidence to further support the IFT140 association with PKD was shown in the study by Chang et al. (2022) that reported a significant association of IFT140 in both those with phenotypic ADPKD (OR 32.94) and in those noted to have any kidney or liver cyst (OR 3.25). Here, our analysis showed that IFT140 LoF variants were significantly more likely to be present in those with cystic disease than those without, providing additional statistical support for an association between LoF variants in IFT140 and PKD.
Of the LoF variants we identified, 64% were not reported in the gnomAD dataset, which is supportive of a lower frequency of these variants in the general population. c.2399 + 1G > T (p.?) was the most common variant identified by both Senum et al. (2022), found in 29.2% (19/65) of pedigrees, and the current study, accounting for 16.6% of the LoF variants identified (Table 1). This variant and the second most common variant in our study, c.1377G > A (p.Trp459*), are documented as present in 14 and 22 alleles in gnomAD, respectively. The relatively high observed carrier rates for these variants in gnomAD may reflect reduced penetrance in a seemingly healthy population, or a possible later onset of disease in individuals with these variants. Although many LoF variants identified here were present in gnomAD, it is important to note that the MAF of each variant reported in our cohort was higher than reported in gnomAD (a primarily healthy dataset), supporting the enrichment of heterozygous LoF IFT140 variants in a primarily diseased cohort. Furthermore, data from gnomAD suggests that IFT140 has a high tolerance for LoF variants (pLI = 0). However, this may be reflective of the mild phenotype and reduced penetrance associated with heterozygous variants, resulting in the inclusion of these individuals in a database that only excludes data associated with dominant, severe pediatric conditions.
It should be considered that variable expressivity could be a factor because in the majority of affected individuals a mild phenotype could go undiagnosed due to lack of cystic proliferation and retained renal function, only reaching a clinically recognizable level later in life. In accordance with the milder phenotype associated with IFT140-related PKD, a small proportion of cases were reported to have late or end stage CKD. Disease progression was noted with increasing age, but considering that half of individuals with PKD1 and PKD2 variants have ESKD by 58 and 79 years respectively, the lower severity of CKD is notable (Cornec-Le Gall et al., 2019). The severity of disease (based on the clinical information provided) in individuals with co-occurrence of IFT140 pathogenic variants and other cystic disease diagnostic findings (n = 8) did not differ from those with heterozygous P or LP variants in only established ADPKD genes; however, this observation is based on a small sample size that is unlikely to find an association.
Limitations to this study included a lack of discrete clinical information as descriptive diagnoses needed to categorize disease into cystic subtypes were often not available. As a retrospective analysis of de-identified test results, this study was limited to clinical and demographic information collected at the time of testing. Additionally, for the protection of patient identities, clinical details were not variant specific and were reported in aggregate. Details of clinical diagnoses and patient phenotypes were commonly limited to ICD-10 codes and/or stage of disease. Given the paucity of clinical phenotypic data, the onset of disease could not be assessed. Likewise, the prevalence of clinically reported cystic disease as well as the diagnostic yield among those with cystic disease may be underrepresented in this study. Interpretation of our results should consider our recruitment approach given all subjects were referred for testing on the basis of having renal disease or a family history of disease. Additionally, other genetic causes of cystic disease, such as SEC61B and DNAJB11, would not be detected in this study due to their absence from this genetic panel. Thus, it is possible that unidentified variants in these other genes could contribute to the cystic presentation for patients in this cohort. For clarity of this study, we excluded all missense variants as functional studies would be necessary to establish a loss of function effect.
This study offers further evidence implicating IFT140 LoF variants in the development of PKD, especially in those cases where no additional molecular etiology has been identified. For cases with a P or LP variant in an established ADPKD gene, the role played by the LoF IFT140 variant may be explained by compounding ADPKD etiologies, a modifying effect, or a necessary component of co-inherited variants. Future work with larger clinically defined cohorts as well as studies investigating the role of variants where the molecular consequence is currently unknown, will allow for clarification of the mechanism of ADPKD development as related to IFT140 variants and the prognosis for those who carry causative variants.
AUTHOR CONTRIBUTIONS
Conceptualization: D.C., L.M.V., J.X., S.P; Data curation: D.C., K.P.H.L., L.L. Y.X; Analysis: D.C., M.S.B., R.B. Writing original draft: D.C, M.S.B.; Writing-review and editing: D.C., R.B., M.S.B., K.P.H.L., L.L., L.M.V., J.X., S.P., Y.X.
FUNDING INFORMATION
Natera Inc. provided funding for data curation, analysis, and preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
D.C., R.B., K.P.H.L., L.L., L.M.V., J.X., M.S.B. and S.P are full time employees of Natera, Inc., with stocks or options to own stocks in the company. Y.X is an employee of and owns stock in Fulgent Genetics.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




