Subtle phenotypes of Mowat–Wilson syndrome in a patient with a novel ZEB2 C-ZF domain variant
To the Editor
ZEB2 encodes the Zfh1 family of 2-handed zinc finger/homeodomain proteins that act as transcriptional repressors of target DNA. Mowat–Wilson syndrome (MWS, MIM number#235730) is caused by heterozygous loss-of-function variants of ZEB2 and is characterized by moderate to severe intellectual disability, congenital heart disease, Hirschsprung disease or chronic constipation, genitourinary anomalies, and distinctive dysmorphic features (Ivanovski et al., 2018). Here, we describe a patient with a ZEB2 missense variant and moderate developmental delay, but without characteristic clinical features of MWS.
The patient was a 3-year-old male and the third child of healthy nonconsanguineous parents after four incidents of spontaneous abortions. He was delivered at 38 weeks gestational age after an uneventful pregnancy, and had a weight of 3431 g (1.0 SD), birth length of 52.5 cm (1.8 SD), and an occipital-frontal head circumference (OFC) of 36 cm (1.9 SD). He controlled his head position at age 4 months, sat at 10 months, stood with support at 12 months, and started walking at 24 months. However, by the age of 3 years and 7 months, he had not spoken any words, and he was referred to our institution for assessment because of developmental delay. At referral, his height was 96.3 cm (−0.3 SD), weight was 14.85 kg (0.0 SD), and OFC was 55.2 cm (1.5 SD). Dysmorphic features, other than slightly flared eyebrows, were not noted (Figure 1a). He showed moderate developmental delay (developmental quotient of 42 at 3 years and 5 months old). Karyotype and chromosomal microarray were normal.
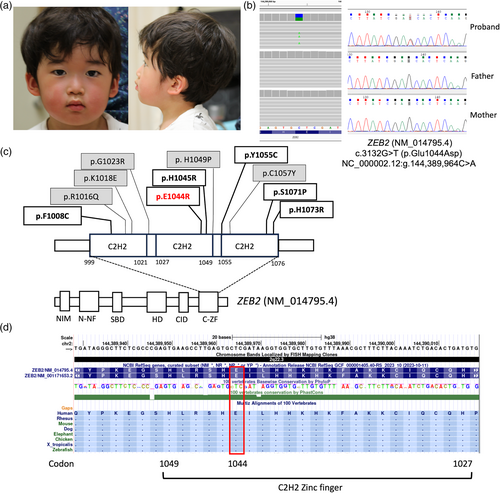
The patient and family members were enrolled in an institutional review board-approved study with informed consent at Kanagawa Children's Medical Center. Finally, trio exome sequencing identified a novel heterozygous variant in ZEB2 (NM_014795.4:c.3132G>T (p.Glu1044Asp) NC_000002.12:g.144,389,964C>A), which was confirmed by trio Sanger sequencing (Figure 1b). The mean depth of coverage was greater than 68.51 per base, and 97.8% of the coding region was covered by at least 10 reads in proband's exome sequencing. The variant is considered “likely pathogenic” according to the 2015 American College of Medical Genetics and Genomics variant interpretation guidelines (PS2_supporting+PM1+PM2+PP3).
In this case report, we describe a patient with moderate developmental delay harboring a de novo heterozygous missense variant in C-ZF domain of ZEB2 gene. The patient lacked distinctive clinical features of MWS, such as congenital heart defects, epilepsy, Hirschsprung disease, and recognizable dysmorphic features. The patients demonstrated very subtle MWS phenotypes including only slightly flared eyebrows. This report suggests that ZEB2 variants also cause nonsyndromic intellectual disability as well as Mowat–Wilson syndrome.
Five missense variants in the C-ZF domain have been identified in six patients that cause atypical MWS without characteristic dysmorphic features other than intellectual disability, developmental delay, and epilepsy (Figure 1c; Table 1) (Amor & Bijlsma, 2018; Ghoumid et al., 2013; Wenger et al., 2014).Characteristic complications of MWS were not present in all of these six patients, including congenital heart disease (0/6), Hirschsprung disease (0/6), and urogenital anomalies (2/6) (Table 1). Other missense variants have been noted inside and outside of the C-ZF domain, but clinical details and their photographs have not been reported. Thus, missense variants of the C-ZF domain may manifest with nonsyndromic intellectual disability without anomalies other than atypical MWS.
| Ghoumid et al. | Wenger et al. | |||||||
|---|---|---|---|---|---|---|---|---|
| This patient | Patient 1 | Patient 2 | Patient 3 | Patient 5 | Patient 7 | Amor et al. | ||
| Genotype ZEB2 NM_014795.4) | c.3132G>T (p.E1044D) | c.3134A>G p.H1045R) | c.3164A>G p.Y1055C) | c.3211T>C p.S1071P) | c.3023T>G p.F1008C) | c.3211T>C p.S1071P) | c.3218A>G p.H1073R) nd 16p11.2 deletion syndrome | |
| Dysmorphic features present in MWS | Mild | Mild | Mild | Typical | Mild | Mild | Mild | Mild 6/7 |
| Widely spaced eyes | N | N | Y | N | N | N | N | 1/7 |
| Broad eyebrows with a medial flare | Y | N | N | Y | Y | N | N | 3/7 |
| Low-hanging columella | N | Y | Y | N | Y | Y | Y | 5/7 |
| Prominent or pointed chin | N | Y | Y | Y | Y | Y | Y | 6/7 |
| Open-mouth expression | N | N | N | Y | N | Y | N | 2/7 |
| Uplifted earlobes | N | N | N | Y | N | N | Y | 2/7 |
| ID/DD | Moderate | Moderate | Moderate | Moderate | Mild | Mild | Severe | 7/7 |
| Microcephaly | N | N | N | N | N | Na | N | 6/6 |
| Motor skill | ||||||||
| Sitting (months) | 10 | 12 | 12 | NA | NA | NA | NA | |
| Walking (months) | 24 | 23 | 24 | 26 | NA | 18 | NA | |
| Epilepsy | N | Y | N | N | N | Y | Drop attack | 3/7 |
| Brain anomaly | NA | Hippocampal anomalies, frontal cortical atrophy | N | N | NA | N | NA | 1/4 |
| Hirschsprung disease | N | N | N | N | N | N | N | 0/7 |
| Constipation | N | N | N | N | Y | Y | Y | 3/7 |
| Cardiac malformation | N | N | N | N | N | N | N | 0/7 |
| Urogenital anomalies | N | Hypospadias | N | N | Lower pole lobulation of the kidney | N | N | 2/7 |
| Others | a(see caption) | |||||||
- Abbreviations: ID/DD, intellectual disability/developmental delay; MWS, Mowat–Wilson syndrome; N, no; NA, not available; Y, yes.
- a Recurrent vomiting, pyloric stenosis, severe eczema, anxiety, obsessive-compulsive disorder, associated with stereotypic behaviors, skin picking and trichotillomania.
Missense variants in the C-ZF domain would be hypomorphic variants affecting binding affinity with target DNA molecules. The C-ZF domain is highly conserved among species and has binding motifs toward target DNA molecules that regulate target gene expression, such as transcriptional factors (Figure 1d) (Remacle et al., 1999). Binding to the E-cadherin gene promotor was decreased in reported ZEB2 missense variants of the C-ZF domain, including p.His1045Arg, p.Tyr1055Cys, and p.His1073Arg (Ghoumid et al., 2013). These variants seemed to have hypomorphic loss-of-function effects, so at least C-ZF domain variants would cause attenuated loss-of-function of ZEB2. Hypomorphic ZEB2 variants lead to mild or subtle MWS phenotypes including developmental delay, intellectual disability, and reminiscent craniofacial features.
In conclusion, ZEB2 C-ZF domain variants are associated with intellectual disability with subtle dysmorphic features of MWS that might result from hypomorphic variants with attenuated loss-of-function effects. Our results suggest a phenotypic spectrum from MWS to non-syndromic intellectual disability in ZEB2-related neurodevelopmental disorders.
AUTHOR CONTRIBUTIONS
Kenji Kurosawa designed the study. Takuya Naruto performed the bioinformatics experiments and contributed to the genetics experiments. Yukiko Kuroda recruited and evaluated the study subjects. Kenji Kurosawa supervised Yukiko Kuroda. Yukiko Kuroda wrote the manuscript.
ACKNOWLEDGMENTS
This study was supported by the Japan Agency for Medical Research and Development (AMED), MHLW Health Labour Sciences Research Grant (number 23FC1052), and JSPS KAKENHI Grant (number 23K14966 to YK). We thank the patient and her family for their cooperation.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




