A novel ABCC9 variant in a Greek family with Cantu syndrome affecting multiple generations highlights the functional role of the SUR2B NBD1
Jian Gao and Athina Ververi contributed equally to this study.
Abstract
Cantu syndrome (CS) (OMIM #239850) is an autosomal dominant multiorgan system condition, associated with a characteristic facial phenotype, hypertrichosis, and multiple cardiovascular complications. CS is caused by gain-of-function (GOF) variants in KCNJ8 or ABCC9 that encode pore-forming Kir6.1 and regulatory SUR2 subunits of ATP-sensitive potassium (KATP) channels. A novel heterozygous ABCC9 variant, c.2440G>T; p.Gly814Trp, was identified in three individuals from a four generation Greek family. The membrane potential in cells stably expressing hKir6.1 and hSUR2B with p.Gly814Trp was hyperpolarized compared to cells expressing WT channels, and inside-out patch-clamp assays of KATP channels formed with hSUR2B p.Gly814Trp demonstrated a decreased sensitivity to ATP inhibition, confirming a relatively mild GOF effect of this variant. The specific location of the variant reveals an unrecognized functional role of the first glycine in the signature motif of the nucleotide binding domains in ATP-binding cassette (ABC) protein ion channels.
1 INTRODUCTION
Cantu syndrome (CS) is a rare autosomal dominant genetic disorder, associated with characteristic coarse facial appearance, hypertrichosis, joint hyperflexibility, lymphedema, and multiple cardiovascular abnormalities, including tortuous blood vessels, patent ductus arteriosus, and cardiac hypertrophy (Grange et al., 2014, 2019). All reported cases of CS are caused by inherited or de novo gain-of-function (GOF) variants in either KCNJ8 or ABCC9. KCNJ8 and ABCC9 encode the Kir6.1 pore-forming and SUR2 regulatory subunits, respectively, of hetero-octameric ATP-sensitive potassium (KATP) channels (Aguilar-Bryan et al., 1998; Shyng & Nichols, 1997) that are found predominantly in muscle tissues (Flagg et al., 2010). There are two major splice variants of SUR2, SUR2A and SUR2B (Chutkow et al., 1996; Shindo et al., 1998). KATP channels composed of Kir6.1 and SUR2B are predominantly expressed in vascular (Brayden, 2002), lymphatic (Davis et al., 2020), and gastrointestinal (York et al., 2020) smooth muscles. KATP channels formed by Kir6.2 and SUR2A are present in cardiac and skeletal muscle (Chutkow et al., 1996; Foster & Coetzee, 2016).
All KATP channels share the same architecture (Li et al., 2017; Sung et al., 2021) and similar modulation of channel function by nucleotides (Nichols, 2006). ATP inhibits the channel by nonhydrolytic binding to a pocket formed at the interface between the Kir6.x cytoplasmic domains (Tucker et al., 1997). SUR subunits are members of the ATP-binding cassette (ABC) transporter superfamily and are composed of TMD0, TMD1, and TMD2 transmembrane domains, with two cytoplasmic nucleotide binding domains (NBDs), between TMD1 and TMD2 (NBD1) and at the very C-terminus of the protein (NBD2). Mg-nucleotide binding to two nucleotide binding sites (NBSs) (Moody et al., 2002) that are formed at the interface between the two NBDs (Puljung et al., 2019; Wu et al., 2018) results in conformational changes that propagate to the channel, leading to channel opening (Nichols et al., 1996). Thus, the activities of KATP channels are determined by intracellular ATP and ADP levels, and KATP channels couple cell metabolism to excitability (Nichols, 2006; Tinker et al., 2018). The nucleotide-bound dimerized NBDs are stabilized by synthesized potassium channel activators such as pinacidil and prevented by channel inhibitors such as glibenclamide (Puljung et al., 2019; Sung et al., 2021).
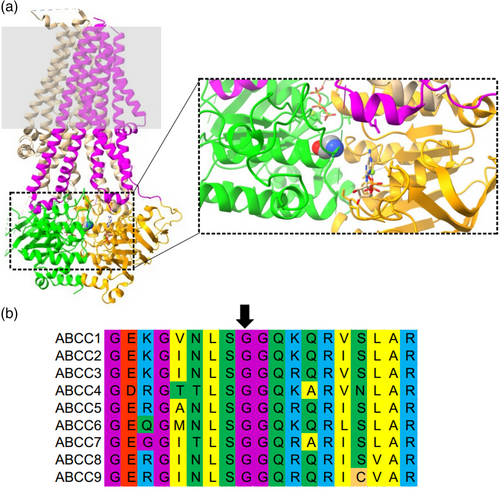
Within the fully dimerized NBDs, NBS1 is formed by the Walker A (“GQVGCGKSS”) and Walker B (“IVFLDD”) motifs in NBD1 and the signature (“FSVGQ”) motif in NBD2, while NBS2 is formed by the equivalent Walker A and B motifs in NBD2 and signature motif in NBD1 (Stockner et al., 2020). NBS2 contains the necessary elements for ATP hydrolysis, including a glutamate residue in the last amino acid in Walker B (Sauna et al., 2002) and conserved “LSGGQ” in the signature motif and is reported to hydrolyze the bound MgATP (Urbatsch et al., 2000). In contrast, NBS1 does not conserve these sequences and has been considered a degenerate NBS that retains MgATP binding but does not support hydrolysis (Ueda et al., 1997). While a full mechanistic understanding remains elusive, there is a consensus that Kir6.x associated with SUR1 or SUR2 subunits are activated by hydrolysis of MgATP to MgADP at NBS2 and that experimentally, addition of MgADP to the cytoplasmic face can maintain the activated SUR state (Masia et al., 2005).
Within multiple members of the ABC transporter family, the conserved Walker B glutamate has been shown to be important in coordinating the γ-phosphate of ATP and the Mg2+ ion and inducing the ATP hydrolysis reaction. Within the LSGGQ motif, mutation of the second glycine has been shown to decrease the activities of several ABC transporters (Bakos et al., 1997; Logan et al., 1994; Szentpetery et al., 2004). The first glycine in the signature motif, which has received less experimental study, is not fully conserved within the ABC family (Stockner et al., 2020), suggesting tolerance to mutation. Here, we report a variant involving the first signature glycine, p.Gly814Trp, in SUR2B that was identified in three generations of a family. The variant segregated with the clinical diagnosis of CS, suggesting a causal effect on KATP channel activity.
2 MATERIALS AND METHODS
2.1 Editorial policies and ethical considerations
Genetic testing was performed as part of clinical diagnosis. Ethical approval was not required. Written informed consent was obtained for publication of all images of family members.
2.2 Molecular genetic analysis
The proband underwent clinical genetic testing on peripheral blood via an intellectual disability gene panel. Genomic DNA was enriched for targeted regions using a hybridization-based protocol and was, subsequently, sequenced at >50× depth using Illumina technology. Reads were aligned to the reference sequence (GRCh37). Genomic DNA was isolated from saliva samples submitted by six additional family members using the Lamda Biotech Animal Genomic Test Kit. Primers (int21F: ATCAGCCTGAAGCCATCTTG and int22R: CAGCACTGGAATGGGGTACT) were ordered (Integrated DNA Technology) flanking the 22nd exon of abcc9 in order to generate a 688-bp product containing the putative Gly814Trp variant. The int21F primer was subsequently used for detection by Sanger sequencing.
2.3 Molecular biology and cell culture
The identified p.Gly814Trp variant was introduced into human SUR2B (Gao, McClenaghan, Matreyek, et al., 2023) (pcDNA3.1-_ hSUR2B; GenBankTM accession no. NP_064693.2) cDNA using site-directed mutagenesis and verified by direct Sanger sequencing. Wild type (WT) and p.Gly814Trp (G814W) hSUR2B cDNA were subcloned into a vector including hKir6.1 and IRES sequence for stable cell line generation using the landing pad HEK293 cell system (Matreyek et al., 2017).
HEK293 stable cells expressing hKir6.1 and WT or G814W hSUR2B were generated as described previously (Gao, McClenaghan, Matreyek, et al., 2023). For transient expression, cosm6 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) and transfected using FuGENE 6 (Roche Applied Science) with wildtype pcDNA3.1_hKir6.2 (0.6 μg; GenBankTM accession no. NG_012446.1) and wildtype or variant pcDNA3.1_hSUR2B constructs (1 μg) in addition to 0.2 μg of pcDNA3.1_eGFP for visual detection of successful transfection. Excised patch-clamp recordings were made 48–72 h posttransfection.
2.4 Membrane potential assessments by voltage-sensitive fluorescent dye
This experiment was conducted as described previously (Gao, McClenaghan, Matreyek, et al., 2023). Briefly, transparent cell-culture 96-well plates were coated with poly-l-lysine (0.1 mg/mL) 1 day before stable cells were plated at an appropriate density. Cells were cultured with 100 μL of DMEM containing 10% FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 4 μg/mL doxycycline. After 1 day in culture, the medium was discarded and the cells were washed twice with low [K] (1 mM) buffer (80 μL/well). Cells were then incubated with 3 μM DiBAC4(3) in low K buffer, with or without additional channel modulators, for at least 20 min. Separate images of green and red fluorescence were taken with EVOS M5000 imaging system (10× lens).
The recipe for low K buffer is: NaCl 139 mM, KCl 1 mM, CaCl2 2 mM, MgCl2 1 mM, HEPES 10 mM, and glucose 10 mM. The pH was adjusted to 7.4 with NaOH.
Exported tiff images were analyzed by CellProfiler with custom designed programs for landing pad cells and generated stable cell lines. Multiple measurements of each cell were exported as a csv format file and the intensities of all cells as well as the mean value in each image were extracted with a custom script written in Python©. The average intensity value of duplicate images was calculated as one data point for the statistical analysis and data presentation.
2.5 Excised inside-out patch-clamp
2.6 Data analyses
All statistical analyses were performed using Microsoft Excel or Prism (GraphPad). Significance values were calculated using Student's t-test. All values are expressed as mean ± SD.
3 RESULTS
3.1 Clinical information
The proband is a 5-year-old boy with developmental delay, dysmorphic features (Figure 1a1), and hypertrichosis. He is the first child of nonconsanguineous parents of Greek (father) and Albanian (mother) origin. He has a maternal half-brother who is healthy. The pregnancy was complicated by mild polyhydramnios, for which no maternal causes were identified. The polyhydramnios was detected at 28 weeks gestation and persisted throughout the third trimester. The pregnancy was otherwise uncomplicated and the proband was born at 40 weeks gestation. His growth parameters at birth were normal (birth weight 20th centile, head circumference 22nd centile, and length 33rd centile) and remained at similar centiles at last follow-up visit at 5 years. The child was referred for genetic assessment due to the diagnosis of autistic spectrum disorder (ASD) and dysmorphic features. The diagnosis of ASD was confirmed with the use of Autism Diagnostic Observation Schedule, second edition (ADOS-2) and Autism Diagnostic Interview-Revised (ADI-R). The proband's developmental delay has gradually improved. He is currently functioning at an almost age-appropriate level and is scheduled to attend mainstream school without support. There were no health concerns at his last follow-up visit at the age of 5 years, although echocardiogram revealed aortic root ectasia (z score 1.98). He is under follow-up by a pediatric cardiologist and a developmental pediatrician.

Due to his constellation of findings, the proband underwent genetic analysis using an intellectual disability gene panel at the age of 3 years (Invitae Daignostic Testing). The testing identified a heterozygous c.2440G>T; p.Gly814Trp variant in ABCC9 which was absent from gnomAD and all in silico tools predicted pathogenicity. Parental testing showed that the father, aged 38 years, also carries the variant, but with no reported health concerns, other than hypertrichosis and cholesteatoma. On physical examination, the father has facial features consistent with CS (Figure 1a2). Both the proband and his father were referred to cardiology, and the father was found to have ventricular hypertrophy and mild aortic dilatation (44 mm, z score 3.3). Segregation testing in the family did not identify the variant in the paternal aunt, who has a normal phenotype. However, the paternal grandmother, who has a CS facial appearance and hypertrichosis, was also found to carry the variant (Figure 1a3). She is 62 years old and does not have any reported health concerns, apart from ventricular hypertrophy. Her father is reported to have had a similar facial appearance, as confirmed by examination of family photographs. He also had a cholesteatoma and died at 67 years of “premature old age.” The family pedigree is shown in Figure 1b.
3.2 The p.Gly814Trp variant increases the basal activity of KATP channels
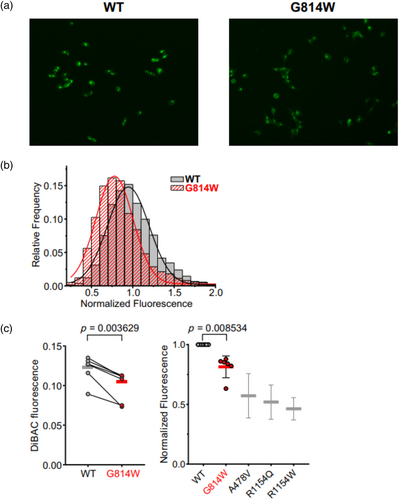
Gly814 in SUR2 is located in NBD1, at the interface of the dimerized NBD1/2 conformation (Figure 2a) and is conserved in all human ABCC family protein sequences (Figure 2b). To examine the effect of the p.(Gly814Trp) on Kir6.1/SUR2B-dependent ion channel activity, the variant was introduced into human SUR2B cDNA and stable cell lines expressing wildtype hKir6.1 and wildtype hSUR2B (WT) or p.Gly814Trp (G814W) mutant hSUR2B were generated as previously described (Gao, McClenaghan, Matreyek, et al., 2023). DiBAC4(3), a cell membrane sensing fluorescent dye assay was used to assess membrane polarization. A representative experiment is shown in Figure 3a, together with the distribution of fluorescence intensity in individual cells (Figure 3b). Under basal conditions, G814W cells exhibit lower mean intensity fluorescence (0.099 ± 0.017) than WT cells (0.121 ± 0.015, Figure 3c), reflecting a membrane hyperpolarization effect that indicates a gain of channel function under basal conditions in intact cells, although the effect is weaker than previously seen for other established CS GOF variants (Gao, McClenaghan, Matreyek, et al., 2023). From these DiBAC4(3) measurements, the relative K conductances were as follows: WT 1.0; G814W 1.8 ± 0.6; A478V 4.4 ± 2.0; R1154Q 5.0 ± 2.0; R1154W 5.8 ± 1.7; calculated using a simplified Goldman–Hodgkin–Katz equation that assumes permeability is conductance and that all non-K conductances can be lumped into a single “sodium” conductance (eq. 6 in Gao, McClenaghan, Matreyek, et al. [2023]).
3.3 The p.Gly814Trp variant decreases sensitivity of recombinant channels to ATP inhibition
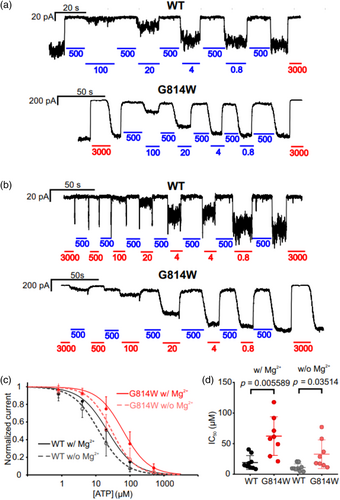
To further assess the effect of the variant on channel activity, inside-out patch-clamp assays were performed on Cosm6 cells transiently transfected with hKir6.2 and WT or G814W hSUR2B, and GFP. Both wildtype and mutant channels were dose-dependently inhibited by ATP in the presence (Figure 4a) or absence (Figure 4b) of physiological (0.5 mM free) Mg2+. Under both conditions, the inhibitory effect of [ATP] was reduced ~3-fold in G814W channels, with IC50 increasing from ~20 to 60 μM in the presence of Mg2+ and from ~10 to ~30 μM in the absence of Mg2+ (Figure 4c,d), with no significant change in the Hill coefficient.
4 DISCUSSION
We provide the first report of a Greek family with CS, in which the genetic basis was confirmed in three generations, but the phenotype was present in at least four generations. The affected individuals show many of the typical features associated with CS, and all harbor a heterozygous c.2440G>T variant in ABCC9 resulting in a p.Gly814Trp variant in NBD1 of SUR2. Functional assays of recombinant KATP channels containing p.Gly814Trp (G814W) exhibit reduced sensitivity to inhibitory ATP compared to WT, providing a molecular explanation of the condition.
4.1 Role of the LSGGQ glycine in ABC protein function
Both the DiBAC4(3) assay and the patch-clamp analyses indicate that the variant p.Gly814Trp increases Kir6.1/SUR2B-dependent channel activity. Naively, it may be predicted that the effect of this variant, being located within NBD1 and at the Mg2+ nucleotide-dependent interface of NBD1/2 dimers would affect channel activity in the presence of Mg2+, by increasing the activatory effects of MgADP, as is seen with many other CS variants in SUR2 (Cooper et al., 2015; Gao, McClenaghan, Christiaans, et al., 2023; McClenaghan et al., 2018). Interestingly, the patch-clamp assay shows that the variant decreased sensitivity to ATP, even in the absence of Mg2+. One possibility is that even in the absence of MgATP and subsequent conformational changes, the NBD dimerization-induced effect is mimicked by this variant. It is also possible that ATP binding in the absence of Mg2+ is increased by this variant, so that the outward-facing (NBD-dimerized) conformation is stabilized (Sikimic et al., 2019). In NBD-dimerized SUR1 Cryo-EM structures (Wu et al., 2018), the NBS2 is not fully closed, with a predicted distance between the Gly814 backbone carbon and the ATP adenine group of ~11 A. Conceivably, the bulky G814W tryptophan side chain may help to close the gap and stabilize the NBD-dimerized conformation. A recent study reported the insertion of a regulatory (R-domain) peptide between the two NBDs of SUR2A, in which the main chain of neighboring Gly815 was proposed to bind to a glutamate residue within the R-domain (Ding et al., 2023). Interestingly, p.Gly815Ala has also been reported in association with CS (Grange et al., 2019), and variant p.Gly832Cys in SUR1 (equivalent to Gly814 in SUR2) has been reported in association with neonatal diabetes mellitus (Yamazaki et al., 2017), which is caused by GOF variants in Kir6.2/SUR1 channels. Conceivably, a mutation of either or both LSGGQ glycines may thus work by destabilizing the R-domain insertion and favoring NBD dimerization. The equivalent G550W mutation in ABCC7 (which generates the CFTR chloride channel) also increases sensitivity to protein kinase A activation and channel open probability (Chen et al., 2023), although mutations at the corresponding site in more distant ABC transporters have been reported to be without functional effect (Kumar et al., 2010; Meier et al., 2023). A potentially simple explanation is that for ion channels CFTR and KATP, just stabilizing NBD dimerization will favor channel opening, whereas both NBD dimerization, hydrolysis, and nucleotide dissociation are required for a complete cycle in ABC transporters.
4.2 Clinical implications
The majority of identified CS individuals are de novo or have at most one or two antecedent affected individuals in any given family (Grange et al., 2019). While this might reflect identification bias resulting from modern exome sequencing being more likely to be carried out in children, it might also suggest that, historically at least, premature mortality resulting from untreated pulmonary and cardiovascular complications (Grange et al., 2014, 2019) precluded the generation of very extensive CS pedigrees. Clearly, complications in neonates or infants account for some premature CS deaths (Grange et al., 2014, 2019), but premature death in adulthood is also likely; there are few reports of older (>50 years) males with confirmed CS and evidence for heart failure in older females with CS (Singh et al., 2022). That said, the penetrance of different CS features has thus far been difficult to predict, and no quantitative molecular phenotype–organism phenotype relationship has been developed. In this regard, it is interesting that the p.Gly814Trp variant underlies an extended pedigree in the present family, with at least four generations affected, and at least one older male and an affected antecedent who died at 65. Possibly, in comparison to other confirmed CS variants (Gao, McClenaghan, Matreyek, et al., 2023), p.Gly814Trp demonstrated a more mild GOF effect in recombinant channels. In future, a quantitative molecular phenotype–organism phenotype relationship may be necessary, particularly for predicting the ability of SUR2-inhibitors to treat CS, since the sensitivity to such inhibitors can be dependent on the severity of the GOF itself (Gao, McClenaghan, Matreyek, et al., 2023; McClenaghan et al., 2018).
A cholesteatoma, an abnormal accumulation of keratin-producing squamous cells in the middle ear, was present in two affected individuals. Cholesteatomas are most often seen in association with chronic recurrent ear infections and are rarely congenital. This feature has not previously been described in CS, and with no obvious connection to the underlying molecular basis, it is uncertain if there is a causal link between CS and cholesteatoma.
AUTHOR CONTRIBUTIONS
Clinical characterization and molecular genetic analysis were carried out by AV, AS, FR, CG, RT, and DKG. Mutagenesis and recombinant ion channel analysis was carried out by JG and ET. The article was initially drafted by JG, AV, and CGN. All authors gave final approval for publication.
ACKNOWLEDGMENTS
These studies were supported by R35 grant HL171542 from the US National Institute of Health (to CGN).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




