A unique case of hyperammonemia due to CA5A deficiency: Impact of coexisting gene mutations, pseudogene, and microdeletion
Abstract
Carbonic anhydrase 5A (CA5A) belongs to a family of carbonic anhydrases which are zinc metalloenzymes involved in the reversible hydration of CO2 to bicarbonate. Mutations in CA5A are very rare and known to cause Carbonic anhydrase 5A deficiency (CA5AD), an autosomal recessive inborn error of metabolism characterized clinically by acute onset of encephalopathy in infancy or early childhood. CA5A also has two very identical pseudogenes whose interference may result in compromised accuracy in targeted sequencing. We report a unique case of CA5AD caused by compound heterozygous variant (NM_001739.2: c.721G>A: p.Glu241Lys & NM_001739.2: c.619-3420_c.774 + 502del4078bp) in an infant in order to expand the phenotypic spectrum and underscore the impact of pseudogenes, which can introduce complexities in molecular genetic analysis.
Abbreviations
-
- CA5A
-
- Carbonic anhydrase 5A
-
- CA5AD
-
- Carbonic anhydrase 5A deficiency
1 INTRODUCTION
Carbonic anhydrases (CAs) represent a family of zinc metalloenzymes that facilitate the reversible hydration of carbon dioxide. These enzymes actively participate in a myriad of biological processes, spanning respiration, calcification, acid–base homeostasis, bone resorption, and the synthesis of crucial bodily fluids such as aqueous humor, cerebrospinal fluid, saliva, and gastric acid (van Karnebeek et al., 2014). Notably, they exhibit pronounced diversity in both their distribution across tissues and subcellular localization. Carbonic anhydrase 5 A (CA5A) is localized within the mitochondria and predominantly expressed in hepatic tissues. CA5A is implicated in pivotal roles associated with ureagenesis and gluconeogenesis. The CA5A gene is present on chromosome 16q24.3, with an unprocessed pseudogene assigned to 16p12-p11.2 (Nagao et al., 1995). Biallelic variants in CA5A cause a rare autosomal recessive inborn error of metabolism (IEM) called CA5A deficiency (OMIM 615751), which clinically manifests as infantile hyperammonemic encephalopathy that is associated with metabolic irregularities such as hypoglycemia, hyperlactatemia, metabolic acidosis, and respiratory alkalosis (Marwaha et al., 2021; Semenova et al., 2022). It is already known that CA5A has two pseudogenes (ENST00000550637.1, ENST00000568175.1), that have almost 95% identity to the actual CA5A gene.
Pseudogenes are genomic DNA sequences that resemble functional genes but have lost their ability to encode functional proteins. They are frequently observed non-coding sequences distinguished by a notable sequence homology to their protein-coding paralogues (Steyaert et al., 2021). The existence of pseudogenes poses a significant challenge in next-generation sequencing (NGS), as their presence can disrupt the accurate identification of variants within the actual genes, leading to the generation of both false positive and false negative variants. This interference might result in compromised accuracy in targeted sequencing, thereby impacting the reliable identification of genetic variations. Co-amplification of the pseudogene can be avoided through careful experimental design and optimization by designing primers specific to the target gene, excluding regions with high homology to pseudogenes (Valadan et al., 2015). In this report, we delineate an exceptional instance of CA5A deficiency observed in a 3-day-old infant. The genetic landscape of this case is marked by the coexistence of various anomalies, including the presence of a pseudogene, a missense mutation, and a concurrent microdeletion.
2 METHODS
2.1 Editorial policies and ethical considerations
This study was approved by the Centre for DNA Fingerprinting and Diagnostics institutional bioethics committee (approval no. IEC 40/2021 dated 7th April 2021).
2.2 Whole mitochondrial genome sequencing analysis
The entire mitochondrial genome (16.5 kb) was amplified from whole blood DNA by long range PCR using PrimeSTAR GXL DNA polymerase (Takara #R050A) with following primers: (1) Set1_F: 5′-GGCTTTCTCAACTTTTAAAGGATA-3′, Set1_R: 5′-TTTATGGGGTGATGTGAGCC-3′; (2) Set2_F: 5′-AACCAAACCCCAAAGACACC-3′, Set2_R: 5′- GCCTCCGATTATGATGGGTAT-3′ and (3) Set3_F: 5′-GCAACCTTCTAGGTAACGACC-3′, Set3_R: 5′-GTGGTAAGGATGGGGGGAATTA-3 to obtain overlapping fragments of lengths 4958, 5585 and 6365 bp respectively. The fragments were pooled in equimolar proportion and library preparation was done using QIAseq FX DNA Library Kit (Qiagen # 180479) according to the manufacturer's protocol. For each sample, paired-end 150 bp reads with average 2500× coverage were obtained. Sequencing reads quality was analyzed using FastQC. Sequencing data was mapped and then aligned to mitochondrial reference genome rCRS (NC_012920.1) using Burrows-Wheeler aligner version 0.7 (BWA-MEM). Mutect2 from Genome Analysis Tool Kit (GATKv.4.1) and Mutserve were used for variant calling. Variants obtained were annotated using the web interface of the MITOMASTER tool. Candidate variants were prioritized by considering the following parameters: (1) All the coding and tRNA variants, (2) variants with allele frequency less than 0.01 in reference populations, such as gnomAD, HelixMTdb, and Mitomap. (3) variants with a heteroplasmy ratio above 5%. (4) deleterious variants potentially affecting the protein and tRNA structure and functions were prioritized based on damage prediction tools MitoTIP and APOGEE.
2.3 WES analysis
Exome capture was done using the Agilent SureSelectXT V5 exome capture kit (Agilent, USA). The library was sequenced to mean 110X coverage on the Illumina HiSeq2000 sequencing platform. The sequences obtained were aligned to the human reference genome (GRCh37/hg19) using the BWA (Burrows-Wheeler algorithm) program and analyzed using Picard and GATK-Lite toolkit to identify variants in the whole exome relevant to clinical indication. Variant annotation was performed using Annovar for location and predicted function. Gross filtering was done using gnomAD (≤0.01 MAF), 1000 genomes (≤0.01 MAF), EVS (≤0.01 MAF), ExAC (≤0.01 MAF) dbSNP, and in-house exome databases. Clinically relevant mutations were annotated using published variants in literature and a set of variant databases including ClinVar, OMIM, and HGMD. Only non-synonymous, splice site, nonsense, and frameshift variants found in the coding regions were used for clinical interpretation. Silent variations that do not result in any change in amino acid in the coding region were excluded. The variant region in proband, father, and mother were confirmed by Sanger sequencing using ABI 3130 Genetic analyzer (Applied Biosystems, USA).
2.4 Quantitative PCR (qPCR)
The qPCR was done to quantify allele copy number in proband, father, mother, and control. 25 ng of genomic DNA and 0.5 pmol CA5A specific primers (CA5A_RT_F: 5′-GCTGGGATTACTGGACCTACGC-3′ and CA5A_RT_R: 5′-TTACGGGCACGGCTCACC-3′) were used. The qPCR reactions were performed using the HiMedia Insta Q96 real-time PCR system and the amplifications were done using the SYBR Green PCR Master Mix (ABI #A25742). The thermal cycling conditions were composed of an initial denaturation step at 95°C for 2 min followed by 40 cycles at 95°C for 15 s and 60°C for 60 s. The experiments were carried out in triplicates for each data point. The relative quantification of gene copy number was determined using the 2−ΔΔCt method. The G6PDH gene with the following primers: G6PDH-F: 5′-TCTTCATCACCACAGAGAACTTGC-3′ and G6PDH-R: 5′-GACCTGGAAGTCACTGGGCA-3′ was used as an internal control for data normalization.
2.5 Long range polymerase chain reaction (LR-PCR)
Long-range PCR was performed to identify CA5A deletions using the following primers: CA5A-del-F: 5′-TCCCATTCTAGCTGCCTGAGGA-3′ and CA5A-del-R: 5′-AGACGAATGCCCTGTTCCTGTACC-3′. The PCR conditions: an initial denaturation at 94°C for 5 min followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 64°C for 15s extension at 68°C for 6 min followed by a final extension at 68°C for 10 min.
3 RESULTS
3.1 Clinical features
The proband was a 3-day-old neonate, born via vaginal delivery to a 32-year-old mother out of non-consanguineous marriage. Father was 35 years old with no significant contributory family history. The neonate was born with a birth weight of 3100 g. She cried immediately at birth not requiring any resuscitation and was discharged home on day 2 of life on breastfeeds. The baby was on exclusive breastfeeds at the time of discharge. On day 3 of life, the neonate was noted to be lethargic and was not accepting breastfeeds. There were also seizures noted, which were multifocal-clonic occurring multiple times. She was referred to the Neonatal Intensive Care Unit (NICU) with complaints of poor feeding, lethargy, and seizures on the day of life 4. At the time of admission, the neonate was having status epilepticus and was stuporous. Vitals signs at the time of admission: temperature: 36.5°C, HR-170 bpm, CFT- 4 s, Peripheral pulses- low volume and poorly felt. RR- 52 bpm, SpO2–92%. These findings were suggestive of shock. Weight at the time of admission was 2655 g with a cumulative 14% weight loss. The head circumference was 34 cm and length 48 cm (normal). There were no dysmorphic features, and no evidence of hepatosplenomegaly, and the rest of the systemic exam including the cardiac examination was normal. Biochemical tests were suggestive of metabolic acidosis with respiratory alkalosis, elevated ammonia, and lactate levels (Table 1). Neonate was stabilized with normal saline fluid bolus followed by intravenous fluids. For further control of shock inotropes (Inj. Dopamine and Inj. Adrenaline were added). She was mechanically ventilated for worsening hemodynamic stability. For the control of seizures, neonate required Inj. Levetericetam, Inj. Phenytoin and Inj. Lorazepam.
| S. Blood sugar | 78 mg/dL (60–125 mg/dL) |
| Ionic calcium | 1.3 mmol/L (0.9–1.1 mmol/L) |
| CBC | Hb-16.5 g/dL, WBC:16,400 per cumm, Platelet: 3.6 lakh/mm3 |
| CRP | 9.5 mg/L (0–10 mg/L) |
| Blood Culture | No growth |
| LFT | T. Bilirubin-4.2 mg%, D. Bilirubin-0.9 mg%, SGOT-58.1 U/L, SGPT-125 U/L |
| S.Ammonia | 281 μmol/L (34–54 μmol/L) |
| INR | 0.9 (0.9–1.4) |
| RFT | Creatinine-0.9 mg/dL (1–2 mg/dL), Urea-24 mg/dL (10–15 mg/dL) |
| S. Sodium | 148 mg/dL (135–149 mg/dL) |
| VBG | pH: 7.335, Pco2-15.5, hco3-8.3, Base deficit: −17.6, Lactate-3.58 mmol/L (Metabolic acidosis with respiratory alkalosis) |
| S.Ketone | 0.22 mmol/L (0–0.6 mmol/L) |
| TMS for organic acidemia and amino acid profile | Within normal limits |
| Lumbar puncture | Protein: 55 mg/dL (19–140 mg/dL), Cells: 0, Gram stain – normal and culture- No growth Normal |
| CPK | 129.4 IU/L (0–130 IU/L) |
| CPK-MB | 205 IU/L (28–300 IU/L) |
| MRI Brain with MR Spectroscopy | White matter hyperintensity noted in globus pallidus with choline peak on MRS |
| USG skull | Normal |
| Eye exam | Normal |
| 2D ECHO | Normal |
| USG Abdomen and KUB | Normal |
- Note: Normal ranges are given in parentheses.
After initial hemodynamic stabilization and control of seizures, neonate was started on mother milk feeds via a nasogastric tube which was gradually incremented and there was no vomiting or episodes of feed intolerance. Neonate was extubated to CPAP support on DOL-10 and subsequently was off oxygen support by DOL-15. Neurologically neonate remained abnormal throughout NICU stay with axial and appendicular hypotonia and poor suck. Neonate was discharged on DOL-40 on cup and spoon feeds and continues to be on Syp. Leveritecetam, Syp. Phenytoin and early intervention.
3.2 Molecular findings
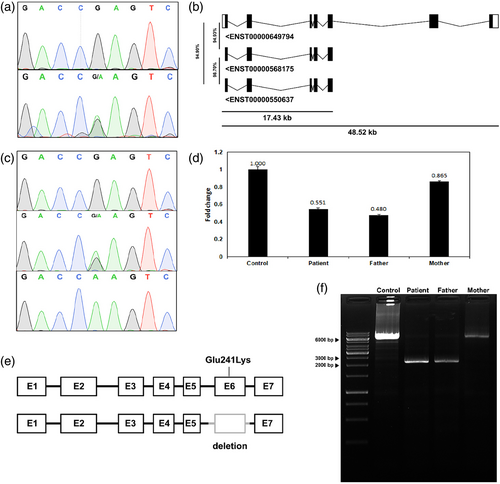
The clinical symptoms of the patient were suggestive of mitochondrial involvement. Hence, we performed a whole mitochondrial genome sequencing of the proband, which revealed no pathogenic or likely pathogenic variant(s). This prompted us to proceed with whole exome sequencing (WES). WES identified a homozygous pathogenic variant (NM_001739.2: c.721G>A: p.Glu241Lys) in exon 6 of the CA5A gene (Figure S1). Interestingly, the variant turned out to be heterozygous on targeted Sanger sequencing (Figure 1a). Upon reanalyzing the exome data, it was found that the sequencing reads also mapped to two highly identical regions (Figure S2) outside the CA5A gene, indicating the presence of a related genomic region that was initially overlooked and later found to be a pseudogene. Two pseudogenes of CA5A were identified on chromosome 16 having a high sequence similarity of almost 95% (Figure 1b). These pseudogenes having high sequence similarity co-amplified along with the target CA5A region, complicating Sanger sequencing and erroneously indicating the variant as heterozygous. By repeating Sanger sequencing with primers specifically designed for CA5A, thereby preventing co-amplification of pseudogenes, the variant was conclusively identified as homozygous in the proband (Figure 1c). Familial segregation analysis concerning the homozygous variant prompted suspicions, pointing towards the potential existence of a microdeletion in the proband's alternate allele which was confirmed using qPCR (Figure 1d). The proband and father were found to harbor a 4078 bp microdeletion (NM_001739.2: c.619-3420_c.774 + 502del4078bp) in the CA5A gene which spans regions of intron 5 and 6, and whole exon 6 (Figure 1e). The deletion breakpoints were identified by long-range PCR (Figure 1f) followed by Sanger sequencing. Therefore, it was confirmed that the proband harbors a pathogenic missense variant in one allele inherited from mother and a microdeletion in the other allele inherited from the father.
4 DISCUSSION
Carbonic anhydrase 5A (CA5A) belongs to a family of carbonic anhydrases which are zinc metalloenzymes involved in the reversible hydration of CO2 to bicarbonate. Fifteen isoenzymes of carbonic anhydrases have been identified, of which only two are known to localize and function in mitochondria. Carbonic anhydrase 5A deficiency (CA5AD) is an autosomal recessive inborn error of metabolism characterized clinically by acute onset of encephalopathy in infancy or early childhood (Olgac et al., 2020) with an age spectrum of 2 days of life to early childhood (20 months). The predominant ethnicity reported so far is of South Asian origin with fewer than 30 cases reported to date (Ganesh & Karthik, 2024; Mani Urmila et al., 2022).
In 2014, van Karnebeek et al. identified the first cases of this disorder, reporting four children from three unrelated families where one family was consanguineous, and exhibited symptoms including lethargy, hyperlactatemia, and hyperammonemia (van Karnebeek et al., 2014). Subsequently, 10 patients were identified to harbor mutations in the CA5A gene among a cohort of 96 patients diagnosed with hyperammonemia (Diez-Fernandez et al., 2016). Miller et al. reported a compound heterozygous mutation involving a maternally inherited out-of-frame deletion spanning exon 3 of CA5A and a paternally inherited CA5A variant, c.473A>C p.(H158P) (Miller et al., 2021). Another study elucidating the association of CA5A deficiency with infantile spasms, expanding the understanding of its neurological phenotype identified a novel homozygous nonsense variation in the CA5A (c.59G > A; pTrp20Ter), leading to premature truncation of the protein causing CA5A deficiency. To date, there are 14 different variants reported to cause CA5A deficiency, out of which 11 are missense mutations and 6 are deletions (Mani Urmila et al., 2022).
We report a distinctive case of hyperammonemia due to CA5AD (OMIM 615751) caused by the coexistence of a pathogenic missense variant along with a microdeletion in the CA5A gene. Molecular diagnosis identified a maternally inherited pathogenic variant (NM_001739.2: c.721G>A: p.Glu241Lys) in exon 6 and a paternally inherited deletion of exon 6 which was missed in the initial WES analysis but was later identified by long-range PCR and quantified by qPCR. Although these variants were previously identified in homozygous condition in several cases of CA5AD, they were never identified in compound heterozygous condition causing the disease. Consistent with the previously reported cases, our patient also presented with a unique combination of biochemical findings, including hyperammonemia, elevated lactate and ketone bodies in urine (Table S1). MRI of the brain showed white matter hyperintensity in globus pallidus along with a choline peak on MRS.
In the present report, we highlighted the case to broaden the understanding of both the mutational and clinical spectrum of the disease and intend to underscore the impact of pseudogenes, which can introduce complexities in molecular genetic analysis.
AUTHOR CONTRIBUTIONS
Rohan Peter Mathew: Conceptualization, methodology, data production, data analysis, writing – original draft. Prashanth Ranya Raghavendra: Data production, data analysis, writing – original draft. Biradar Disha: Methodology, data production, writing – original draft. Ashwin Dalal: Conceptualization, methodology, funding acquisition, writing – review and editing. Periyasamy Govindaraj: Conceptualization, methodology, supervision, funding acquisition, writing – review and editing.
ACKNOWLEDGMENTS
The authors thank Dr. Anitha Haribalakrishna, Associate Professor and Head, Department of Neonatology, Seth GS Medical College and KEM Hospital, Mumbai, for neonatology inputs. R.P.M. and B.D. acknowledges the Council of Scientific and Industrial Research (CSIR), and the Department of Biotechnology (DBT), GoI for their fellowship; respectively. P.G. and A.D. acknowledge the Department of Biotechnology, Government of India (Project ID BT/PR45460/MED/12/952/2022) for funding. The work was carried out as part of the Mission Program on Pediatric Rare Genetic Disorders (PRAGeD).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




