Leukoencephalopathy with calcifications, developmental brain abnormalities and skeletal dysplasia due to homozygosity for a hypomorphic CSF1R variant: A report of three siblings
Abstract
We report three siblings homozygous for CSF1R variant c.1969 + 115_1969 + 116del to expand the phenotype of “brain abnormalities, neurodegeneration, and dysosteosclerosis” (BANDDOS) and discuss its link with “adult leukoencephalopathy with axonal spheroids and pigmented glia” (ALSP), caused by heterozygous CSF1R variants. We evaluated medical, radiological, and laboratory findings and reviewed the literature. Patients presented with developmental delay, therapy-resistant epilepsy, dysmorphic features, and skeletal abnormalities. Secondary neurological decline occurred from 23 years in sibling one and from 20 years in sibling two. Brain imaging revealed multifocal white matter abnormalities and calcifications during initial disease in siblings two and three. Developmental brain anomalies, seen in all three, were most severe in sibling two. During neurological decline in siblings one and two, the leukoencephalopathy was progressive and had the MRI appearance of ALSP. Skeletal survey revealed osteosclerosis, most severe in sibling three. Blood markers, monocytes, dendritic cell subsets, and T-cell proliferation capacity were normal. Literature review revealed variable initial disease and secondary neurological decline. BANDDOS presents with variable dysmorphic features, skeletal dysplasia, developmental delay, and epilepsy with on neuro-imaging developmental brain anomalies, multifocal white matter abnormalities, and calcifications. Secondary neurological decline occurs with a progressive leukoencephalopathy, in line with early onset ALSP. Despite the role of CSF1R signaling in myeloid development, immune deficiency is absent. Phenotype varies within families; skeletal and neurological manifestations may be disparate.
1 INTRODUCTION
Colony stimulating factor 1 receptor (CSF1R)-related leukoencephalopathy is a microgliopathy caused by pathogenic variants in the CSF1R gene. Mono-allelic CSF1R variants lead to adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP, OMIM #221820), characterized by progressive behavioral, cognitive and motor decline and seizures, typically with onset between ages 30 and 55 years and death up to 30 years after onset (Adams et al., 2018; Wong et al., 2011). Bi-allelic hypomorphic and amorphic CSF1R variants have been linked to a leukoencephalopathy with osteosclerosis and clinical presentation between birth and the third decade, called “brain abnormalities, neurodegeneration, and dysosteosclerosis” (“BANDDOS”, OMIM #618476) (Daghagh et al., 2023; Dulski et al., 2023; Guo et al., 2019; Kindis et al., 2021; Monies et al., 2017; Oosterhof et al., 2019; Tamhankar et al., 2020). BANDDOS is characterized by dysmorphic facial features, limb deformities and fractures, developmental delay, and seizures. Neurological decline occurs with dysarthria, ataxia, spasticity and extrapyramidal signs (Daghagh et al., 2023; Dulski et al., 2023; Guo et al., 2019; Kindis et al., 2021; Monies et al., 2017; Oosterhof et al., 2019; Tamhankar et al., 2020). In BANDDOS, initial development may also be normal, with later onset psychiatric symptoms, motor and cognitive decline, and seizures (Chitu et al., 2022).
The overarching term “CSF1R-related leukoencephalopathy” has been proposed to encompass both (Dulski et al., 2023), but does not allow distinction between the dominant and recessive disease.
CSF1R signaling is required for production, differentiation, function and survival of myeloid cells, including microglia, osteoclasts, monocytes, and dendritic cells (DC) (Rojo et al., 2019). In BANDDOS, it has been suggested that impaired CSF1R signaling causes depletion of osteoclasts and microglia in early development, leading to sclerosing skeletal dysplasia, and in the brain developmental brain anomalies, white matter abnormalities, and ectopic calcium deposits (Guo et al., 2019; Oosterhof et al., 2018; Oosterhof et al., 2019). Whether impaired CSF1R signaling also results in deficits of other myeloid cells and dysregulation of the growth hormone and insulin-like growth factor axis, as is observed in Csf1r deficient animal models, (Dai et al., 2002; Hume et al., 2020; Joseph et al., 1999; Keshvari et al., 2021; Pridans et al., 2018) is unknown.
A genotype–phenotype association based on CSF1R gene dosage has been suggested, where bi-allelic hypomorphic variants may lead to an incomplete phenotype without brain developmental anomalies and no or mild skeletal abnormalities (Chitu et al., 2022). We report three siblings with BANDDOS and review the 21 thus far reported patients to enhance the understanding of clinical, radiological, and laboratory features of the disease.
2 METHODS
2.1 Standard protocol approvals and patient consents
This study was approved by the local Institutional Review Board (protocol number: 2018.300). Clinical information was derived from medical records.
2.2 Genetic examination
For sibling one, whole-genome sequencing was performed and analyzed, as described (Helman et al., 2020). For siblings two and three, Sanger sequencing was performed as described in the Supporting information (Data S1).
2.3 Radiological examination
Brain CT was performed in sibling three. Brain MRI at 3 T in all siblings included axial T2- and T1-weighted images and sagittal T1-weighted images, which were assessed for gray and white matter changes, as described (van der Knaap et al., 1999). Gradient echo and susceptibility-weighted images (SWI) were checked for calcifications. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC)-maps were inspected for restricted diffusion, with ADC values below 70 × 10−5 mm2/s. Magnetic resonance spectroscopy (MRS) was performed at 3 T, as described (Steenweg et al., 2016).
Standard x-ray images of the skull, spine, arms, and legs were obtained in siblings two and three.
2.4 Blood laboratory examination
Cell count, liver and renal function, osmolality, lipid profile, circulating IGF-1, and serum neurofilament light chain (NfL) were assessed. Peripheral blood mononuclear cells (PBMCs) of siblings two and three were tested for presence of monocytes (CD88 + CD1c-cells) and DC subsets according to a previously described protocol, (Beerepoot et al., n.d.) included in the Supporting information (Data S1).
3 RESULTS
3.1 Case histories
Sibling one was a 25-year-old male, born after full-term uneventful pregnancy. He had a delayed development with independent walking at 2.5 years, speech delay and learning difficulties. At 23 years, he fell from his bicycle and experienced leg pain since. There were no signs of (old) bone fractures. X-rays revealed thoracic scoliosis and biconcave thoracic vertebrae. From then on, his motor function declined. He developed generalized tonic–clonic seizures. Nine months later, he had lost the ability to walk and speak. Examination at age 25 years revealed a long face, prominent forehead, high-arched palate, low set ears, and dysmorphic hands with short fingers. Height was normal for age. He communicated with gestures. He had right-sided facial weakness, left-sided atrophy, and left deviation of the tongue. He had spasticity of arms and legs, right more than left, without intentional motor function of the right arm and hand and both legs. Gross and fine motor skills of the left arm and hand were slow and accompanied by intention tremor. Reflexes were hyperactive, especially on the right, with bilateral Babinski signs. He died of pneumonia at age 28 years.
Sibling two was a 18-year-old female with unremarkable pregnancy, birth, and neonatal period. From the age of 4 months, she experienced generalized seizures that were difficult to control with medication. Her development was delayed, and she started to speak some words from the age of 3 years and achieved independent walking at age 5 years. Her cognitive development stagnated at a cognitive age of approximately 4 years based on the Dutch Wechsler Preschool and Primary Scale of Intelligence-third edition (WPPSI-III-NL). She was incontinent of urine and feces. At the age of 20, neurological decline set in, with progressive walking and balance difficulties. Her overall health was unremarkable, and she had no history of bone fractures or systemic immunological disease. Upon first examination at 18 years of age, she had no dysmorphic features and height was normal for age. Comprehension was poor. Neurological examination revealed mild pyramidal signs, with hyperreflexia and bilateral Babinski signs, and a spastic gait. No cranial nerve abnormalities or cerebellar signs were noted. Examination at 21 years revealed pseudobulbar dysarthria, more severe pyramidal signs and intention tremor, left more than right. Tandem gait was only possible with support.
Sibling three was a 13-year-old male with a medical history of intracranial hemorrhage and hydrocephalus during pregnancy, and premature birth at 35 + 2/7 weeks gestation. After birth, he had brittle bones with fractures of both arms and legs. From the age of 1 year, he suffered from therapy-resistant epilepsy. He achieved independent walking at age 2 years and spoke his first words at age 3 years. His cognitive age was estimated at 4 years based on WPPSI-III-NL, but he continued to make developmental progress at special education. He was incontinent of urine and feces. He had a clavicular fracture at age 5 years, possibly after a fall. Examination at 13 years revealed mild dysmorphic features, including telecanthus, stocky built, short and stubby fingers and toes, and mild hypertrichosis. Height was normal for age. Comprehension and language production were poor. Neurological examination was unremarkable except for a clumsy tandem gait.
The parents of the siblings were first cousins, of Arabic origin from Lebanon. They had no neurological disease or bone deformities at ages 58 and 49 years. They had two more, unaffected, sons, and no family history of neurological disease.
3.2 Brain MRI and CT
Three brain MRIs in sibling one, obtained between 23 and 25 years, showed progressive multifocal and confluent lesions in the frontoparietal white matter, left more than right, affecting the periventricular, deep and subcortical white matter and corpus callosum, and prominently involving the pyramidal tracts in the posterior limb of the internal capsule and entire brainstem (Figure 1a). Some lesions showed diffusion restriction (Figure 1b, c). No calcifications were found. The lateral ventricles were mildly enlarged and dysplastic.
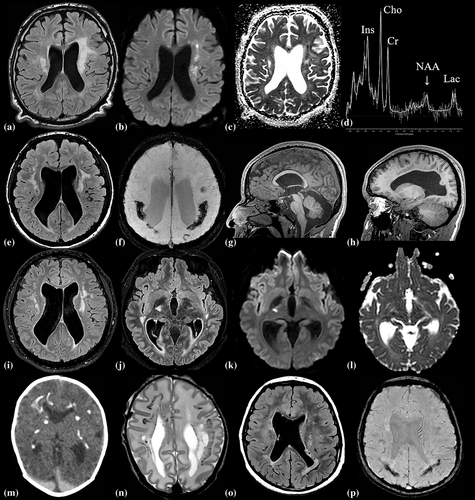
First brain MRI in sibling two at age 18 years revealed wide-spread multifocal frontoparietal white matter lesions and involvement of the pyramidal tracts in the posterior limb of the internal capsule and brain stem. The lateral ventricles were moderately enlarged and dysplastic (Figure 1e). Extensive calcifications were observed, most prominent in the deep parietal white matter (Figure 1f). No diffusion restriction was seen. Developmental brain abnormalities included mega cisterna magna, cavum septi pellucidi, bilateral subependymal cysts, dysplastic fornixes, dysplasia of the pituitary gland and stalk, abnormal migration of the anterior pituitary, and abnormal gyration in the frontomesial and supraoptic regions (Figure 1g,h). Brain MRI at 21 years revealed stable lesions in the frontoparietal white matter and pyramidal tracts (Figure 1i) and novel lesions in the right temporo-pontine tract with diffusion restriction (Figure 1j,l).
In sibling three, neonatal brain CT and MRI (Figure 1m,n) revealed calcifications in the frontoparietal periventricular, deep and subcortical white matter and posterior limb of the internal capsule. The lateral ventricles were asymmetrically enlarged and dysplastic. MRI at 2 years showed extensive confluent lesions in the periventricular and deep white matter and the wide-spread calcifications. MRI at 13 years showed more limited multifocal white matter lesions, mainly in the parietal periventricular region, and the known calcifications (Figure 1o,p). No diffusion restriction was seen.
Proton MRS in sibling one revealed no cortical abnormalities, but strongly decreased N-acetyl aspartate in the abnormal white matter, especially in the lesions located in the centrum semiovale, where N-acetyl aspartate was almost absent (Figure 1d). In addition, slightly reduced creatine and increased choline, myo-inositol, and lactate were observed in the white matter. In siblings two and three, MRS was unremarkable during initial disease and neurological decline (sibling two).
3.3 Skeletal findings
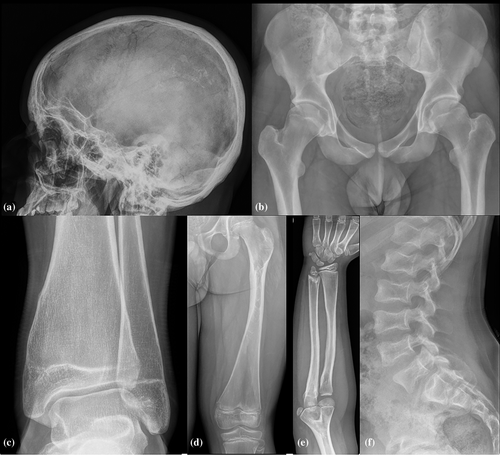
Skeletal surveys in siblings two and three revealed subtle osteosclerosis of the skull base, pectoral girdle, pelvic girdle, and most prominent in the diaphysis of the tubular bones, resembling findings of dysosteosclerosis-Pyle disease spectrum (Figure 2a,b). Both siblings had under-modeling of the tubular bones with relatively radiolucent metaphysis and relatively sclerotic diaphysis, and Erlenmeyer flask deformity of the distal femur and tibia (Figure 2c,d). Skeletal age corresponded with calendar age. In sibling three, the distal ulna showed an irregular metaphysis, resulting in negative ulnar variance (Figure 2e). He had six lumbar-type vertebrae with 11 rib-bearing thoracic vertebrae and one pair of hypoplastic cervical ribs. The thoracolumbar vertebrae presented a biconcave aspect with endplate sclerosis (Figure 2f).
3.4 Genetic testing and blood laboratory results
In sibling one, CSF1R variant screening on the whole-genome sequencing dataset revealed homozygosity of the variant c.1969+115_1969+116del, previously reported in BANDDOS (Guo et al., 2019). In siblings 2 and 3, Sanger sequencing confirmed homozygosity of the same variant, whereas parents were heterozygous. The two healthy siblings were not tested.
Blood laboratory results were normal, except for slightly increased serum creatine kinase (CK) levels in siblings two (277 U/L) and three (313 U/L) (Table S1). On follow-up, serum NfL increased from 7.5 to 14.6 pg/mL in sibling two. Flow cytometric analysis of the PBMCs in siblings two and three confirmed normal absolute and relative numbers of monocytes, albeit with reduced CSF1R expression, and presence of all DC subsets (Figure S1a). CD4 and CD8 T-cells exhibited normal proliferative capacities (Figure S1b). Gating strategy is shown in Figure S2.
3.5 Review of previously published patients
Tables S2 and S3 and Figure S3 summarize findings in our and previously published cases with BANDDOS. Clearly, the initial disease course is variable, even within families, ranging from normal development and appearance to developmental delay, intellectual disability, seizures, dysmorphic features, and skeletal abnormalities. Most patients exhibit osteosclerosis and platyspondyly, with normal body height and no immune disorders. All patients experience secondary neurological decline with spasticity, with onset ranging from 2 to 28 years. Brain imaging during neurological decline reveals white matter abnormalities and calcifications in all patients, with also dilated and dysplastic ventricles, hypoplasia or agenesis of the corpus callosum in most, and other developmental brain anomalies in some patients.
4 DISCUSSION
We describe three siblings with BANDDOS. Initial disease course was marked by neurological and skeletal abnormalities, including developmental delay, epilepsy, and mild osteosclerosis; manifestations were of variable severity and disparate within siblings. Secondary neurological decline occurred in two siblings in their early twenties and had not manifested in the third sibling at age 13 years. No clinical or laboratory evidence of growth or immune deficits was found. Our results align with existing literature, indicating that BANDDOS is characterized by a variable initial disease course, also within families, with neurological and skeletal manifestations but no growth or immunological deficits, and secondary neurological decline in line with early-onset ALSP.
In the initial, stable disease, our patients had bilateral multifocal white matter abnormalities with periventricular and subcortical calcifications. Neonatal imaging in sibling three confirmed that calcifications and white matter abnormalities may be present at birth and may be extensive (Guo et al., 2019;Monies et al., 2017; Oosterhof et al., 2019). The siblings had enlarged dysplastic lateral ventricles. Sibling two had additional developmental brain anomalies, most of which have previously been observed in BANDDOS (Monies et al., 2017; Oosterhof et al., 2019). Animal models suggest that the developmental anomalies and white matter abnormalities might result from microglia dysfunction or depletion in early brain development, disrupting oligodendrocyte differentiation, myelinogenesis, and interneuron migration (Oosterhof et al., 2019). The wide-spread cerebral calcifications could result from ectopic calcification due to osteoclast dysfunction or depletion (Keshvari et al., 2021).
During secondary decline, siblings one and two showed progression of the white matter abnormalities with foci of diffusion restriction, as previously reported in three other siblings, two of whom deteriorated clinically (Kindis et al., 2021). In sibling one, MRS abnormalities were compatible with axonal loss, demyelination, reactive gliosis, and macrophage/microglia activation. These MRI and MRS findings are compatible with progressive ALSP (Bender et al., 2014; Sundal et al., 2012).
Our study suggests that BANDDOS is initially marked by developmental disturbances, with structural anomalies and stable or decreasing white matter lesions, suggesting deficient but ongoing myelination. The subsequent decline has clinical and MRI features of ALSP, although with an earlier onset and more rapid decline than typically seen. Unfortunately, no autopsy was conducted in the first sibling, which could have offered tissue evidence of confirming the typical features of ALSP.
Our cases with mild osteosclerosis showed slightly elevated serum CK levels, similar to a BANDDOS case with osteopetrosis (Oosterhof et al., 2019). While elevated CK levels are linked to osteoclast dysfunction in some sclerosing bone disorders, (Whyte et al., 1996) the significance of this slight increase in BANDDOS remains uncertain. Our findings of normal height, skeletal age, and circulating IGF-1 and lipid profile in blood support normal growth in patients, contrary to Csf1r deficient animal models (Chitu & Stanley, 2017; Dai et al., 2002; Erblich et al., 2011; Hume et al., 2020; Pridans et al., 2018; Ryan et al., 2001). Notably, these are gene knockout models, whereas our patients were homozygous for a hypomorphic variant, suggesting differences in the effects of amorphic versus hypomorphic variants.
Despite the role of CSF1R signaling in myeloid development and cellular and clinical effects observed in Csf1r deficient animal models, (Dai et al., 2002; Hume et al., 2020; Joseph et al., 1999; Keshvari et al., 2021; Pridans et al., 2018; Rojo et al., 2019) including impaired DC differentiation, (Durai et al., 2018) the siblings had no systemic immune disease. Our study revealed reduced CSF1R expression in PBMCs but normal cell numbers, including all DC subsets, consistent with prior research and ALSP (Beerepoot et al., n.d.; Guo et al., 2019; Hofer et al., 2015). Normal T-cell proliferation indicated a functional antigen response.
Homozygosity for the c.1969+115_1969+116del, p.(Pro658Serfs*24) variant was first found in five family members with BANDDOS, exhibiting similar variation in clinical severity of skeletal and neurological abnormalities as our cases. All experienced secondary neurological decline in their teens and twenties, resulting in spastic quadriplegia, mutism, and a vegetative state, compatible with early-onset ALSP (Guo et al., 2019). Neurological decline in sibling one developed rapidly with times from onset to loss of ambulation and death of 1 and 5 years, respectively. In sibling two, neurological decline developed at a similar pace. Most previous cases exhibited slower decline, even those with a much younger age at onset (Guo et al., 2019; Kindis et al., 2021; Oosterhof et al., 2019). Exceptions are two siblings with an onset at the ages of 2 and 4 years and loss of ambulatory function within 6 and 1 months, respectively (Tamhankar et al., 2020). Despite their young age and rapid decline, they did not present with developmental delay and epilepsy, and had no developmental brain or skeletal anomalies (Tamhankar et al., 2020). Like in ALSP, age at onset of neurological decline does not appear to be a strong predictor of disease course.
A potential genotype–phenotype association based on CSF1R gene dosage has been suggested, with homozygosity or compound-heterozygosity for an amorphic CSF1R variant resulting in developmental brain abnormalities, osteopetrosis, and hypocalcaemia, and bi-allelic hypomorphic variants leading to a milder phenotype with brain white matter abnormalities and atrophy, no or mild osteosclerosis, and no brain developmental anomalies (Chitu et al., 2022). Our cases had, similar to other hypomorphic cases, (Erblich et al., 2011; Guo et al., 2019; Kindis et al., 2021) mild osteosclerosis with normal serum calcium levels. However, the presence of developmental brain anomalies argues against a strong correlation between phenotype and CSF1R gene dosing. Additional factors might influence disease severity, e.g. compensation of CSF1R signaling by other cytokine pathways, leading to inter- and intrafamilial variability. Although our and previous findings in family members of patients with BANDDOS suggest that mono-allelic hypomorphic or amorphic CSF1R variants do not cause disease (Daghagh et al., 2023; Dulski et al., 2023; Guo et al., 2019; Kindis et al., 2021; Monies et al., 2017) and argue for a dominant-negative mechanism in ALSP, (Dai et al., 2002) a recent article describes two patients with clinical ALSP despite compound heterozygous CSF1R variants, (Dulski et al., 2024) advocating for the use of the overarching term “CSF1R-related leukoencephalopathy” with subcategories of early (<18 years) and late (≥18 years) onset forms (Dulski et al., 2023).
No published guidelines exist for disease monitoring or therapy in BANDDOS. Alongside neurological examination, surveillance of white matter lesions and restricted diffusion on brain MRI, as well as evaluation of MRS abnormalities, may be considered for disease monitoring. Therapy should focus on supportive care. Hematopoietic stem cell therapy (HSCT) shows promise in restoring CSF1R signaling in ALSP (Eichler et al., 2016; Gelfand et al., 2020; Tipton et al., 2021) and merits further exploration for preventing, ameliorating, or halting secondary neurological decline in BANDDOS. Symptom stabilization after HSCT in a patient with clinical ALSP and bi-allelic CSF1R variants might be considered as the first successful HSCT in BANDDOS (Dulski et al., 2024). However, caution is warranted with offering this intense treatment to severely impaired patients. Compensating CSF1R deficiency with a TREM2 agonist, currently under investigation for symptomatic ALSP (NCT05677659), may serve as an alternative therapy when the secondary decline occurs in BANDDOS.
To conclude, BANDDOS results in variable developmental disturbances of the skeletal and central nervous system and secondly an early-onset ALSP, but not in clinical immune deficits or skeletal growth impairment. Phenotype can vary within families and severity of skeletal and neurological manifestations can be disparate within an individual. We could not confirm that phenotype correlates with age at onset or CSF1R gene dosing. Whether phenotype depends on residual CSF1R signaling in distinct cell types or on other factors remains to be elucidated. Brain MRI and MRS aid in disease monitoring, and emerging therapies for ALSP could potentially prevent or halt the secondary neurological decline in BANDDOS.
AUTHOR CONTRIBUTIONS
S.B and M.v.d.K defined the study theme. S.B, N.I.W, M.v.d.K, J.I.M.L.V, P.J.W.P, and C.S collected the clinical and radiological data and samples. Cell cultures were performed by M.P. Supervision of the study was provided by M.v.d.K and S.N. The first draft of the manuscript was written by S.B, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This study was funded by the WKZ Foundation, grant number D-19-012573. The authors of this publication affiliated with the Amsterdam Leukodystrophy Center are members of the European Reference Network for Rare Neurological Diseases-Project ID No. 739510.
Open Research
DATA AVAILABILITY STATEMENT
Pseudonymized individual data in this article will be made available on reasonable request from a qualified investigator, after approval of the Institutional Review Board.




