Sleep correlates of behavior functioning in Cornelia de Lange syndrome
Abstract
Pathogenic variants in the cohesin genes, NIPBL and SMC1A, both cause Cornelia de Lange syndrome (CdLS), a rare genetic disorder associated with developmental delay and intellectual disability. This study aimed to compare sleep behaviors across individuals with CdLS caused by a variant in NIPBL or SMC1A, and identify relationships between sleep and behavior functioning. A total of 31 caregivers of individuals with a variant in NIPBL (N = 22) or SMC1A (N = 9) completed questionnaires regarding their child's sleep behaviors, behavior regulation, attention, and autistic features (repetitive behaviors and social communication difficulties) as part of the Coordination of Rare Diseases (CoRDS) registry. Findings showed a trend of increased behavior regulation difficulties and repetitive behaviors in the NIPBL compared to the SMC1A participants. Both groups presented with a similar degree of attention, social communication, and sleep challenges. In the whole sample, sleep disturbance was strongly correlated with more behavior regulation difficulties, a relationship that was more robust in the NIPBL sample. In brief, study results support our prior observations of greater behavior difficulties among those with a variant in NIPBL as compared to SMC1A. Preliminary findings point to unique associations between sleep and behavior regulation in the NIPBL group, suggesting sleep interventions may yield differential effects on behavior management across variants.
1 INTRODUCTION
Cornelia de Lange syndrome (CdLS; MIM 122470) is a rare genetic disorder caused by a variant in genes of the cohesin protein complex (NIPBL, SMC1A, RAD21, HDAC8, BRD4, ANKRD11, SMC3) that subsequently results in dysregulated gene expression (Kline et al., 2018). Common features of the syndrome include abnormal growth, microcephaly, developmental delay, limb malformation, and craniofacial differences (Kline et al., 1993; Kline, Grados, et al., 2007). The vast majority of individuals with CdLS have an intellectual disability, with most falling into the profound severity range (50%), followed by severe (24%), moderate (15%), and mild (11%) (Oliver et al., 2008).
Behavioral difficulties are often seen in affected individuals (Mulder et al., 2017), with prevalence rates ranging ~10%–65% for anxiety (Basile et al., 2007; Berney et al., 1999; Gualtieri, 1991; Kline, Grados, et al., 2007), 25%–50% for attention problems (Basile et al., 2007; Berney et al., 1999), and 20%–90% for self-injurious behaviors (Basile et al., 2007; Gualtieri, 1991; Mulder et al., 2017; Srivastava et al., 2014). Behaviors related to autism spectrum disorder (ASD) are observed in most individuals with CdLS. Studies using direct and/or informant-based assessments show an estimated 50%–85% of their sample endorse behaviors that met the clinical cut-off for ASD (Basile et al., 2007; Berney et al., 1999; Bhuiyan et al., 2006; Moss et al., 2008; Moss et al., 2012; Nakanishi et al., 2012; Oliver et al., 2008; Srivastava et al., 2014).
Notably, aberrant sleep behaviors and disordered breathing have also been observed in patients with CdLS. Sleep problems affect around 10%–85% of affected individuals with rates varying based on the instrument used to index abnormal sleep characteristics and the typology of the sleep disorder (Basile et al., 2007; Berney et al., 1999; Hall et al., 2008; Li et al., 2022; Sarimski, 1997; Stavinoha et al., 2011; Zambrelli et al., 2016). Severe insomnia has been seen in 75% of pediatric patients with CdLS and 33% of affected adults (Rajan et al., 2012), which may also contribute to the greater tendency for sleepiness or somnolence during the day (Stavinoha et al., 2011). In a prior investigation utilizing validated informant-report inventories of sleep features, symptoms concerning a sleep breathing disorder were also reported in a little over a third of the sample with CdLS, an elevated rate compared to the general population (Stavinoha et al., 2011). A recent systematic review suggests about 25% of those with CdLS meet criteria for a sleep breathing disorder, and about 50% of affected individuals meet another form of sleep disorder (Bergeron et al., 2020). In effect, behavior regulation challenges and sleep difficulties are pervasive in this condition, which may have a significant impact on their quality of life.
Importantly, the large heterogeneity in clinical presentations associated with CdLS has led to postulations that the genetic disorder manifests in a spectrum ranging from classic to atypical syndrome phenotypes, with less typical clinical profiles possibly associated with variants in genes not yet identified and not associated with cohesin functioning (Kline et al., 2018). Specifically, pathogenic variants in NIPBL have been linked to both the classic and non-classic forms of CdLS (Ansari et al., 2014; Kline et al., 2018), while the other identified genes (SMC1A, SMC3, HDAC8, RAD21, BRD4, ANKRD11) are associated with a generally milder phenotype (Ansari et al., 2014; Deardorff, Bando, et al., 2012; Gil-Rodríguez et al., 2015; Olley et al., 2018). Non-classic forms of CdLS often present with less severe growth delays (Deardorff et al., 2007; Gil-Rodríguez et al., 2015; Mannini et al., 2013) and variable intellectual impairment (Deardorff, Wilde, et al., 2012; Gil-Rodríguez et al., 2015). To date, there have been limited investigations focused on genotype–phenotype associations in behavioral functioning, particularly in sleep. In addition, it remains unclear if sleep disturbances may be associated with the increased behavioral challenges associated with CdLS, given such relationships have been found in many other neurogenetic syndromes including Prader–Willi syndrome and Down syndrome (Cotton & Richdale, 2006; Trois et al., 2009), and sleep is known to impact emotion and behavior regulation (Phillips et al., 2020). Elucidating the unique associations between sleep and behavior functioning across genotypes is an essential step toward personalized care management, given that the etiology of sleep disorders is likely highly disease- and gene-specific (Blackmer & Feinstein, 2016).
To cover this gap in the literature, this study examines behavior regulation, attention/hyperactivity problems, autism-related behaviors, and sleep disturbances across 31 individuals with CdLS due to a pathogenic variant in NIPBL (N = 22) or SMC1A (N = 9). Data included in this study stem from caregiver-informant inventories completed as part of the Coordination of Rare Diseases (CoRDS) registry at Sanford Research. Given that many NIPBL variants have been linked to more severe clinical/physical features of CdLS (Kaur et al., 2023; Kline et al., 2018), we hypothesized that the NIPBL variant group would endorse more sleep and behavior problems than those with SMC1A variants. In line with prior studies focused on sleep in neurodevelopmental disorders (Blackmer & Feinstein, 2016; Robinson-Shelton & Malow, 2016), we anticipated significant correlations between sleep and behavior functioning; however, we had no a priori hypotheses regarding whether the association would differ across two causal genes.
2 METHODS
2.1 Clinical samples and procedures
This study included data from 31 individuals with CdLS residing in the United States. Of the reported causal pathogenic variants, 22 had one in NIPBL and 9 in SMC1A. All data were accessed from CoRDS (https://research.sanfordhealth.org/rare-disease-registry). Of note, the specific variant and inheritance were not information collected as part of the registry. This study primarily involved surveys completed by caregivers of affected individuals with CdLS. These inventories were designed through combined efforts between the CdLS Foundation's clinical and scientific advisors and CoRDS. These were relatively brief to reduce the time commitment for respondents, in efforts to increase registry enrollment, promote standardization in data collection, and afford a bank of natural history information that clinical scientists can access as pilot data while planning for more comprehensive prospective investigations. This study protocol was approved by the Institutional Review Board at Johns Hopkins Medicine.
2.2 Materials
2.2.1 Behavior functioning
The survey completed included 12 items pertaining to behavior regulation, attention problems, and restricted and repetitive behaviors (RRB). Each item required the respondent to indicate if the behavior was ever endorsed: 0 = never a problem; 1 = not a problem today but was in the past; or 2 = currently a problem. Three items were related to communication skills, specifically verbal and nonverbal communication and relatedness with others. These items were rated on a four-point scale reflecting the level of difficulty in the respective area: 0 = no difficulty; 1 = mild difficulty; 2 = moderate difficulty; 3 = severe difficulty. Ratings for the 12 items were first dichotomously coded (0 = not a current problem; 1 = current problem), then aggregated into three composite scores reflective of current symptom endorsement: behavior regulation, attention, and RRB. Ratings for the three items were summed into one index score, social communication difficulties, which indexed symptom severity.
2.2.2 Sleep functioning
Respondents were instructed to indicate if a physician had ever provided a diagnosis of sleep apnea (yes, no, unsure), and if the participant takes prescription or over the counter medication to facilitate sleep (yes, no). Six items related to current sleep disturbances were included in the survey, specifically to assess for challenges with falling asleep, staying sleep, falling asleep after wakening, awakening in the morning, excessive daytime sleepiness, and daytime sleep attacks. These items were rated on a five-point scale (0 = never; 1 = rarely; 2 = occasionally; 3 = frequently; 4 = always) and added together to yield a sleep problems total score. Of note, composite scores were only computed for those who completed all items for a respective domain. For example, if a respondent completed three out of the four items for attention domain, a composite score was not generated. Tables 1 and 2 include the number of respondents across NIPBL and SMC1A groups who completed all items sufficient to yield index scores.
| NIPBL variant (N = 22) | SMC1A variant (N = 9) | t-value or FET, p-value | |
|---|---|---|---|
| Behavior regulation difficulties (% of sample reporting symptom as a current problem) | |||
| Has temper outbursts or tantrums | 12/20 (60%) | 1/8 (12.5%) | FET p = 0.038 |
| Demands have to be met immediately | 12/19 (63.2%) | 1/8 (12.5%) | FET p = 0.043 |
| Isolates self from others | 3/19 (15.8%) | 1/8 (12.5%) | n.s |
| Difficult to reach, contact, or get through | 6/17 (35.3%) | 1/7 (14.3%) | n.s. |
| Behavior total (minimum to maximum score = 0–4) | M = 1.52, SD = 1.17, N = 17 | M = 0.57, SD = 0.97, N = 7 | t = 2.05, p = 0.06 |
| Attention (% of sample reporting symptom as a current problem) | |||
| Excessively active | 7/17 (41.2%) | 2/8 (25%) | n.s. |
| Restless | 10/18 (55.6%) | 4/7 (57.1%) | n.s. |
| Does not pay attention to instructions | 10/17 (58.8%) | 4/7 (57.1%) | n.s. |
| Easily distractible | 10/17 (58.8%) | 6/8 (75%) | n.s. |
| Attention total (minimum to maximum score = 0–4) | M = 2.40, SD = 1.72, N = 15 | M = 2.14, SD = 1.34, N = 7 | n.s. |
| Restricted and repetitive behavior (RRB) (% of sample reporting the behavior as a current problem) | |||
| Rocks body back and forth | 3/19 (15.8%) | 1/8 (12.5%) | n.s. |
| Spins, twirls, or paces | 8/19 (27.7%) | 0/8 (0%) | FET p = 0.063 |
| Need to line up items or make symmetrical | 4/19 (21.1%) | 1/8 (12.5%) | n.s. |
| Unable to throw things away | 1/17 (5.9%) | 0/8 (0%) | n.s. |
| RRB total (minimum to maximum score = 0–4) | M = 0.94, SD = 1.08, N = 17 | M = 0.25, SD = 0.46, N = 8 | T = 2.22, p = 0.03 |
| Social communication difficulties (% of sample reporting behavior as a moderate to severe problem) | |||
| Relate to people and having relationships | 2/19 (10.5%) | 1/8 (12.5%) | n.s. |
| Verbal communication | 12/18 (66.7%) | 7/7 (100%) | FET p = 0.001 |
| Nonverbal communication | 5/18 (27.8%) | 3/8 (37.5%) | n.s. |
| Autism feature total (minimum to maximum score = 0–9) | M = 4.16, SD = 1.91, N = 18 | M = 4.50, SD = 1.22, N = 6 | n.s. |
- Note: Total scores represents only the subset of participants with ratings for all domain items.
- Abbreviations: FET, Fishers exact test; n.s., not significant results (p > 0.10).
| NIPBL variant (N = 22) | SMC1A variant (N = 9) | t-value or FET, p-value | |
|---|---|---|---|
| Sleep apnea (% of sample with reported diagnosis) | 3/19 (15.8%) | 1/17 (11.1%) | n.s. |
| Sleep medication/aid (% of sample on a medication for sleep disturbance) | 5/20 (25%) | 4/8 (50%) | n.s. |
| Sleep disturbances (% of sample reporting frequently to always affected by the problem) | |||
| Falling asleep | 1/18 (5.6%) | 3/9 (33.3%) | n.s. |
| Staying asleep | 3/18 (26.7%) | 3/9 (33.3%) | n.s. |
| Falling asleep after waking up during the night | 3/18 (26.7%) | 2/9 (22.2%) | n.s. |
| Awakening early | 4/18 (22.2%) | 4/8 (50%) | n.s. |
| With excessive sleepiness in daytime | 3/19 (15.8%) | 1/9 (11.1%) | n.s. |
| Daytime sleep attacks | 0/16 (0%) | 0/8 (0%) | n.s. |
| Sleep problems total minimum to maximum score = 0–24) | M = 7.06, SD = 4.62, N = 16 | M = 7.57, SD = 7.25, N = 7 | n.s. |
- Note: Total scores represents only the subset of participants with ratings for all domain items.
- Abbreviations: FET, Fishers exact test; n.s., not significant results (p > 0.10).
2.3 Data strategy
Data analyses were conducted with SPSS 29.0. Independent t-tests were used to examine group differences in behavior regulation, attention, RRB, social communication difficulties, and sleep problems indexes. Fishers exact test was applied to determine group differences in sociodemographic variables and the proportion of patients endorsing specific behaviors, diagnosed with sleep apnea, and placed on medication to address sleep challenges.
Bivariate correlations were utilized to examine the relationship between sleep problems and behavior regulation, attention, RRB and social communication difficulties in the whole sample. Subsequently, correlational analyses were repeated separately for NIPBL and SMC1A groups to determine if the identified relationships between sleep problems with attention and behavior regulation differ across genotype. Of note, as outlined below, the associations between ASD-related behaviors (RRB, social communication difficulties) with sleep problems were not explored in the variant groups given that the correlation did not reach significance in the whole sample. To correct for multiple tests, an alpha cut-off of 0.0125 was applied.
3 RESULTS
3.1 Clinical sample
Table 3 outlines the participant characteristics, including the sociodemographic information collected. Racial composition and participants' insurance coverage, which was considered a proxy for socioeconomic status, were comparable across the NIPBL and SMC1A groups. The majority of both groups comprised of white participants with private or commercial insurance. Although no group effect was observed for the age of participants, the NIPBL group had a significantly larger standard deviation or variance (NIPBL age range in years: 3–36, SMC1A range: 6–15). All participants reside in the United States.
| NIPBL variant (N = 22) | SMC1A variant (N = 9) | t-value or FET, p-value | |
|---|---|---|---|
| Sex | 12F | 5F | n.s. |
| Age (year) (mean (standard deviation)) | 13.82 (11.56) | 9.56 (3.35) | n.s. |
| Race | |||
| White | 86.4% | 77.8% | n.s |
| Asian | 4.5% | 22.2% | |
| African-American | 4.5% | 0% | |
| Other | 4.5% | 0% | |
| Insurance | |||
| Private/commercial | 70% | 75% | n.s. |
| Medical assistance | 15% | 12.5% | |
| Military | 15% | 12.5% |
- Abbreviations: FET, Fishers exact test; n.s., not significant results (p > 0.10).
3.2 Behavioral functioning and autism-related features
Table 1 outlines the proportion of the NIPBL and SMC1A participants who endorsed behavior regulation difficulties, attention problems, and autism-related features (RRB, Social Communication). We observed an overall trend of increased symptoms of behavior regulation difficulties and RRB in NIPBL group as compared to participants with a SMC1A variant (effect size estimates: behavior regulation, d = 0.85; RRB, d = 0.73). Specifically, a larger proportion of the NIPBL variant group endorsed a history of temper tantrums, an urgent need for demands to be met immediately, and stereotyped behaviors (spins, twirls, and paces) as compared to the SMC1A group. Although both variant groups were mostly comprised of individuals with verbal communication difficulties, the proportion of those in the SMC1A group (100%) exceeded that of the NIPBL group (66.67%). Overall, the two groups yielded similar scores in attention problems and social communication difficulties.
3.3 Sleep functioning
As shown in Table 2, both the NIPBL and SMC1A groups endorsed a similar severity of sleep problems and reported a comparable proportion of participants with diagnosed sleep apnea and medication use for sleep dysfunction. Within the NIPBL group, difficulties with sleep maintenance, falling back asleep after waking, and awakening early were most commonly reported (~20%–25% of the sample across items). A large proportion of the SMC1A group also endorsed these sleep characteristics, in addition to problems with sleep initiation (20%–50% of the sample across items). A relatively large number of participants were on medication for sleep dysfunction. Approximately 25% of those in the NIPBL group reported a need for pharmaceutical intervention, compared to 50% of the SMC1A group. Although a group effect was not seen in medication use, it is likely the low sample sizes reduced statistical power and sensitivity to detect differences.
3.4 Associations between sleep and behavior functioning
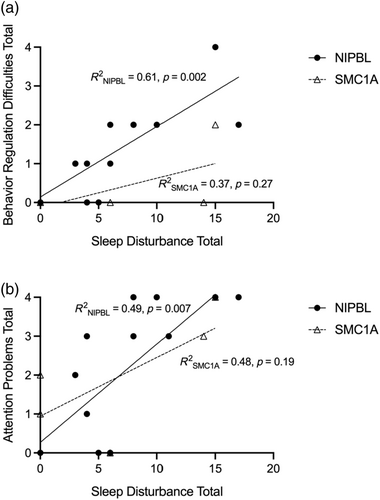
In the whole sample, sleep disturbance was strongly correlated with behavior regulation difficulties (r(18) = 0.64, p = 0.004, r2 = 0.40) and attention problems (r(18) = 0.68, p = 0.002, r2 = 0.46), but not autism-related behaviors (RRB, social communication difficulties). When the relationships were examined across the variant groups, sleep disturbance was strongly correlated with behavior regulation difficulties (r(13) = 0.78, p = 0.002, r2 = 0.61) and attention problems (r(13) = 0.70, p = 0.007, r2 = 0.49) in the NIPBL group, but these associations did not reach significance for the SMC1A group, providing preliminary evidence of unique sleep correlates with behavior as a function of variant status (Figure 1a,b).
4 DISCUSSION
This study represents the first investigation to explore genotype and sleep phenotype in CdLS and the associations between behavior and sleep dysfunction, two commonly affected domains among those with the syndrome. The main findings highlight more behavior regulation and repetitive behaviors in those with NIPBL variants, but comparable attention and sleep challenges across NIPBL and SMC1A variants. Sleep disturbances were associated with greater behavior regulation and attention difficulties in the whole sample, albeit more strongly in the NIPBL group. In effect, interventions directed at sleep in individuals with CdLS may have a shared effect on behavior management, but particularly so among individuals with the NIPBL variant.
Emergent evidence has suggested a differential behavioral phenotype among individuals with a variant in NIPBL versus SMC1A (Ng et al., 2024). Importantly, the NIPBL variant has been associated in general more with a classic CdLS phenotype, that is, more severe cognitive impairment and developmental delay (Deardorff, Wilde, et al., 2012; Gil-Rodríguez et al., 2015; Kaur et al., 2023). Our results implicate that those with an NIPBL variant may also share a more severe behavioral phenotype, represented by more behavioral challenges such as elevated attention difficulties and RRBs, than that in individuals with a variant in SMC1A. Given that RRBs and symptoms of attention-deficit hyperactivity disorder have been linked to perturbations in the cortical-basal ganglia circuitry (Lewis & Kim, 2009; Riva et al., 2018), our findings may also suggest different developmental trajectories in brain maturation associated with NIPBL as compared to SMC1A variants. However, to our knowledge, the literature focused on comparing the neurobehavioral profile across CdLS-related variants by use of standardized data collection is extremely limited, and thus, more investigative work in this area with larger samples and combined application of behavioral and neurobiological metrics (e.g., standardized clinical observation assessments, caregiver-reported inventories, and neuroimaging studies) are warranted.
Notably, our results somewhat diverge from those reported by Moss et al. (2017), who led a genotype–phenotype investigation examining behavior functioning in those with NIPBL versus non-NIPBL variants. Those authors did not report differences in mood, anxiety, impulsivity, inflexible behaviors, and autism spectrum characteristics between those with a variant in NIPBL versus the NIBPL-negative group. However, discrepancies across study findings may stem from the heterogeneous comparison sample incorporated in Moss et al. (2017), as those with variants in HDAC8 and SMC1A were all clustered into the same group (non-NIPBL). Similarly, our study was constrained by the genetic information collected by the CoRDS registry, which does not require participants to submit molecular confirmation of the syndrome (e.g., genetic test findings) to maintain anonymity. As such, molecular factors (e.g., frameshift, missense, etc.) were not accounted for in our study. Accordingly, it is possible that the NIPBL versus SMC1A groups had different compositions of truncating versus non-truncating variants that drove our observed patterns.
In our study, both NIPBL and SMC1A variants have an increased risk of sleep problems reported, with over 20% of each group endorsing at least one sleep disturbance. In the same vein, a sizeable proportion of both groups reported taking medication to facilitate better sleep, albeit no group differences emerged in statistical testing. Although no group effects were observed, both participant groups reported higher rates of pediatric sleep apnea than reported estimates from the general population (Marcus et al., 2012), consistent with findings from a recent investigation examining sleep patterns in CdLS broadly (Li et al., 2022). Overall, findings continue to implicate that sleep dysfunction may be prominent among those with the syndrome (Stavinoha et al., 2011), supporting the need to integrate assessments of sleep regularly among clinicians who work with patients with CdLS. Both behavioral and pharmacologic treatments may be considered to optimize sleep, which in turn may improve quality of life. Prospective research with mixed measures, including polysomnograms and behavioral indices such as a sleep journal, is needed to better characterize whether different gene variants associated with CdLS may be associated with certain classifications of sleep disorders, warranting a genotype-focused approach in treatment planning (e.g., surgical intervention such as tonsillectomy, pharmacological therapy, or behavioral approaches).
Preliminary findings from our study suggest that the relationship between sleep and behavioral phenotypes may differ across variants associated with CdLS, supporting recent efforts in designing syndrome- or disease-specific screening and management (Abel & Tonnsen, 2017; Blackmer & Feinstein, 2016) and integration of preventive education with caregivers to promote sleep practices (Mindell & Meltzer, 2008; Mindell & Owens, 2015). However, several factors that were unaccounted for in our study should be considered in future work. It is plausible that the varying severity of the CdLS clinical phenotype mediate the associations between sleep and behavior functioning. For example, although seizures occur more among individuals with CdLS than the general population, loss of function variants in SMC1A are associated with a greater occurrence (45%) than NIPBL (15%) (Kline et al., 2018). As such, seizure disorders may explain the increased verbal communication deficits reported in our SMC1A sample and may potentially impact behavior regulation more robustly than sleep disturbance. Similarly, another limitation in our data collection and interpretation is the lack of information regarding participant's gastrointestinal history, including history of gastroesophageal reflux disease, and its relationship with sleep and behavior functioning across CdLS-related variants. It is known that 80% of individuals with CdLS have reflux, with greater prevalence observed in those with an NIPBL variant (70%) than with an SMC1A variant (60%) (Kline et al., 2018; Parma et al., 2020). Sleep disorders and other behaviors including self-injurious behavior are known to be affected by reflux, especially in those with NIPBL variants (Zambrelli et al., 2016). In brief, this study did not control for other neurological and gastrointestinal characteristics of CdLS, which also should be considered in subsequent investigations.
In addition, given that CdLS has been associated with some evidence for accelerated aging including physical changes such as graying of hair, facial changes, and early onset of osteoporosis (Kline, Krantz, et al., 2007), longitudinal study designs and a developmental approach will be vital in delineating the genotype–phenotype associations. At present, it remains unclear if specific variants are linked to more rapid aging, and whether this extends to cognition and behavior. However, some investigations on genotype and behavioral phenotype in CdLS have shown older age is uniquely associated with more inflexible behaviors among those with NIPBL variants (Moss et al., 2017). Others have also shown individuals with a clinical diagnosis of CdLS show more disruptive behaviors, anxiety, and antisocial behaviors with age (Basile et al., 2007). While there are some clinical observations of improved sleep with age in CdLS (Kline et al., 2018), there has been no empirical evidence to support these trends. It is imperative for clinical investigators to consider a developmental framework when assessing the dynamic interactions between sleep and behavioral health, as the relationships may modify over time, warranting different care management approaches with varying maturational periods.
4.1 Study limitations and future directions
Although our study provides novel findings about sleep and behavioral patterns in CdLS, several study limitations should be considered. In addition to the concerns noted above, our study had small sample sizes, resulting in low statistical power and poor sensitivity to identify group effects in sleep and behavioral attributes. The surveys administered as part of the patient registry included limited items on behavioral functioning and sleep, and thus, our assessment of behavioral regulation, attention, autism features, and sleep dysfunction was not comprehensive in nature. In turn, we were unable to assess for different typologies of sleep, behavior, and attention disorders. The use of standardized inventories that have been validated for clinical use in populations with neurodevelopmental disorders such as intellectual disability should be applied in future research efforts. Lack of control samples (e.g., individuals with intellectual disability without a genetic syndrome and age-matched typically developing individuals) also limited our ability to contextualize findings. Consequently, while our study results offer clues for future directions in deep phenotyping across CdLS variants, data interpretation should be considered with caution.
In brief, results from this study implicate comparable sleep dysfunction across those with NIPBL versus SMC1A pathogenic variants but greater behavioral difficulties in those with NIPBL variants. Increased sleep disturbance is associated with more behavior regulation and attention problems in CdLS, with stronger effects seen in those with NIPBL variants. Assessment and management of sleep will be important to regularly consider when working with individuals with CdLS, as promoting sleep health can impact behavior and improve overall quality of life.
AUTHOR CONTRIBUTIONS
J.O. and A.D.K. helped draft the original survey questions. R.N. conceived the study and designed the project. R.N. wrote the first draft of the manuscript with feedback from A.D.K. D.S. helped coordinate the collection of surveys from the families. All authors read, edited, and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank the Cornelia de Lange Syndrome Foundation for initiating the project, the patients, and their families who participated in this study and acknowledging the support of Coordination of Rare Diseases (CoRDS) registry at Sanford Research.
FUNDING INFORMATION
R.N. is supported by grants from the Wiedemann-Steiner Syndrome Foundation and KAT6 Foundation. R.N. also receives support from the Intellectual and Developmental Disabilities Research Center at Kennedy Krieger Institute and Johns Hopkins University, National Institute of Health grant (NIH P50HD103538).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




