Early diagnostic clues of mucolipidosis type II: Significance of radiological findings
Abstract
Mucolipidosis type-II (ML-II) is an ultra-rare disorder caused by deficiency of N-acetylglucosaminyl-1-phosphotransferase enzyme due to biallelic pathogenic variants in GNPTAB gene. There are a few known about the natural history of ML-II. In this study, we presented the natural course of 24 patients diagnosed with ML-II. Mean age at diagnosis was 9.3 ± 5.7 months. All patients had coarse face, developmental delay, and hypotonia. The mean survival time was 3.01 ± 1.4 years. The oldest patient was 6.5 years old. Twelve patients died due to lung infection and respiratory failure. We observed early and significant radiological findings of ML-II were different from typical dysostosis multiplex such as femoral cloaking, rickets-like changes, and talocalcaneal stippling. These are significant findings observed in the fetal or newborn period which is considered to be highly characteristic of ML-II and disappears in the first year. Cloaking, rickets-like changes, and stippling were not observed in patients older than three months of age and this suggests that these findings disappear within the first year. These radiological features can be used as important clues for diagnosis. We detected eight different pathogenic variants in GNPTAB gene, three of them were novel.
1 INTRODUCTION
Mucolipidosis type II (ML-II) is autosomal recessively inherited, ultra-rare metabolic disorder. It is caused by deficiency of the N-acetylglucosaminyl-1-phosphotransferase (GlcNAc-1 phosphotransferase) enzyme (Reitman et al., 1981). ML-II is caused by the complete deficiency of the GlcNAc-1 phosphotransferase enzyme whereas the partial deficiency of this enzyme is detected in ML-III (Cathey et al., 2010; Raas-Rothschild et al., 2000). This enzyme is required for the production of mannose-6-phosphate (M6P), which is the identifier for lysosomal enzymes (Hasilik et al., 1981; Reitman et al., 1981). Since lysosomal enzymes without recognition markers cannot enter the lysosome, the extracellular levels of enzymes increase unlike other lysosomal storage diseases. GlcNAc-1 phosphotransferase have three subunits as alpha, beta, and gamma. Alpha and beta subunits are encoded by GNPTAB gene. The GNPTAB gene is located on the long arm of chromosome 12 (Raas-Rothschild et al., 2000; Tiede et al., 2005).
ML-II is a severe lysosomal storage disorder with early-onset and progressive clinical manifestations. Its prevalence is estimated between 1:100,000 and 400,000. It is characterized by coarse face, gingival hyperplasia, macroglossia, cardiac involvement, visceromegaly, umbilical and inguinal hernias, short stature, and skeletal anomalies such as thickened cranium, pectus carinatum, pectus excavatum, kyphosis, scoliosis, joint contractures, hip dysplasia, and pes equinovarus. On the radiographs, J-shaped sella, vertebral body malformations, oar-shaped costa, hip dysplasia, enlargement of iliac wings, narrowed iliac bodies, long bone shortening, widened metaphyses, bullet-shaped phalanx that overall have been termed as dysostosis multiplex are observed. In early infancy, radiographic features may resemble hyperparathyroidism and rickets due to periosteal “cloaking” and ossification delay (David-Vizcarra et al., 2010). Severe cardiac and respiratory problems are the primary cause of death which is expected in the first decade (Edmiston et al., 2019).
There are no curative treatments available for ML-II. Treatment is mainly supportive such as physiotherapy, orthopedic surgery, bilevel positive airway pressure support (BIPAP), vitamin and mineral supplementation, feeding support with percutaneous endoscopic gastrostomy (PEG), pharmacological or surgical treatment targeting cardiac functions, and hernia surgery. For this reason, it is crucial to describe the natural history of ML-II which may shed light on early diagnostic findings and enable future therapeutic interventions, such as gene therapy. In this study, it is aimed to delineate age at symptom onset, presenting symptoms, radiological findings, survival, and genotypic features of patients with ML-II.
2 MATERIALS AND METHODS
2.1 Study design and patients
This is a retrospective and descriptive study. Thirty patients who were followed up with the diagnosis of ML-II in the Department of Pediatric Metabolism and Nutrition, Çukurova University Faculty of Medicine between 2010 and 2022 were evaluated. Sociodemographic findings, age at symptom onset, diagnosis and death, surgical history, physical examination findings, neurological evaluation, laboratory and radiological findings, and genetic analysis were recorded. Urine glycosaminoglycan could not be measured in all patients due to technical problems. The patients were diagnosed by either elevated plasma activity of lysosomal enzymes (α-mannosidase and β-hexosaminidase A + B) or molecular analysis of the GNPTAB gene. Lysosomal enzyme analysis (α-mannosidase and β-hexosaminidase) in leukocyte was performed by fluorimetric method. The study was approved by the ethics committee of Çukurova University, Adana, Turkey.
2.2 DNA extraction and variant analysis
Informed parental consent was obtained for all patients in accordance with the ethical standards of the institutional ethical committee (Cukurova University, Faculty of Medicine Non-Invasive Clinical Research Ethics Commission) and the Helsinki Declaration.
Two milliliters peripheral blood samples were collected from patients who were referred to Cukurova University Department of Medical Genetics of Medicine. Genomic DNA isolation were performed using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) via QIAcube automatized system, according to the manufacturer's instructions. The quality of DNA samples was assessed with a Qubit Fluorimeter 3.0 (Thermo Fisher Scientific, Waltham, MA, USA).
The next-generation sequencing workflow was performed to achieve a minimum of 100× coverage on an Illumina MiSeq (Foster City, CA, USA) platform is custom-designed by our center's GNPTAB gene panel including all exons, introns, and exon–intron junctions.
Raw data (FASTQ) were processed using CLC Genomics Workbench (QIAgen, Hilden, Germany) and quality control parameters were checked for both sequencing and variant qualities. Total yield, sequencing quality score, depth of coverage, the quality score of variants, forward/reverse read balance, population, and variant frequencies were assessed as primary variant analysis. Detected variations were evaluated and confirmed via Integrative Genome Viewer (IGV, version 2.8.2). Variants were categorized based on their pathogenicity according to the American College of Medical Genetics (ACMG) criteria as pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, and benign. Additionally, variant analyses were made in comparison with HGMD, 1000 Genome Frequency and Ingenuity Knowledge Base databases. In silico analysis tools, including SIFT, B-SIFT, Polyphen-2, MutationTaster, BLOSUM, PROVEAN, CADD, DANN, GeneSplicer, PhyloP, MaxEntScan, and QCI Inferred Activation were also used for the further examination of the VUSs.
2.3 Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics for Windows version 23.0 (IBM Corp., Armonk, NY, USA). The normality of distribution of numerical variables was evaluated using the Kolmogorov–Smirnov test. Continuous variables were presented as means ± standard deviation or medians (minimum [min] − maximum [max]) according to distribution of data, and categorical variables as number and percentage (%).
3 RESULTS
3.1 Clinical findings
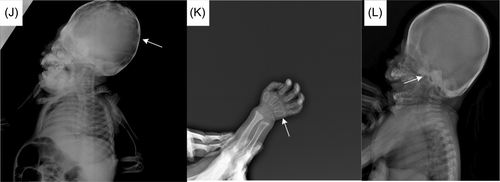
Twenty-four patients were diagnosed with ML-II. Female/male ratio was 15/9. Twenty-two patients had parental consanguinity. Six patients had positive family history. Mean age at symptom onset was 2.7 ± 1.39 months. Mean age at diagnosis was 9.3 ± 5.7 months. The mean diagnostic delay was 6.5 ± 5.6 months. The patients were admitted with hypotonia, facial appearance, recurrent infections, respiratory distress, and bone fractures. Interestingly, one patient was referred from the newborn screening program with suspicion of phenylketonuria, coarse facies, and hypotonia were noticed later on follow-up. One patient was referred with suspicion of skeletal dysplasia after birth due to intrauterine shortened long bones. Ten patients had intrauterine growth retardation (IUGR). None of the patients' medical records showed any other pregnancy-related complications. On physical examination, mean height standard deviation scores (SDS): −2.4 ± 1.0, weight SDS: −2.8 ± 0.6. All patients had coarse face (Figure 1), narrow forehead, long philtrum, developmental delay, and hypotonia. Six patients had craniosynostosis. While 11 patients had gingival hypertrophy at first admission, gingival hypertrophy developed in nine patients during follow-up. Corneal clouding was detected in 18 patients. Fourteen patients had short neck and six had hypertrichosis. Hepatomegaly was detected in eight patients. None of them had splenomegaly. Most of patients (15/24) had inguinal and/or umbilical hernia. Fourteen patients had joint stiffness and ten had pectus carinatum. Clinical findings of patients are summarized in Table 1.

| # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | M | M | F | M | F | M | F | F | M | M | F | F |
| Age at first symptoms (months) | 2 | 3 | 2 | 2 | 3 | 3 | 5 | 1 | 3 | 3 | 3 | 3 |
| Age at diagnosis (months) | 3 | 7 | 3.5 | 3 | 18 | 7 | 12 | 4 | 7 | 11.5 | 9 | 10 |
| Current age (months) | 45 | − | − | − | − | 78 | − | − | 44 | − | − | − |
| Parenteral consanguinity | + | + | + | + | + | + | + | + | + | + | + | − |
| Family history | + | + | − | − | − | − | − | − | − | − | + | + |
| IUGR | − | + | + | + | − | + | − | − | − | − | − | − |
| Weight SDS | −3.04 | −2.87 | −2.12 | −3.13 | −2.8 | −1.76 | −2.95 | −3.66 | −2.1 | −3.87 | −2.74 | −1.79 |
| Height SDS | −1.22 | −3.95 | −2.13 | −2.01 | −2.61 | −1.27 | −2.36 | −3.27 | −2.2 | −4.86 | −2.47 | −2.72 |
| Developmental delay | + | + | + | + | + | + | + | + | + | + | + | + |
| Coarse face | + | + | + | + | + | + | + | + | + | + | + | + |
| Corneal clouding | − | + | − | − | + | + | + | + | + | + | + | + |
| Gingival hyperplasia | + | + | + | + | − | + | + | + | + | + | − | + |
| Short neck | + | + | − | − | − | + | − | − | − | − | + | − |
| Chest deformity | + | + | − | − | − | + | − | − | + | + | + | − |
| Cardiac findings | − | − | − | − | + | + | + | − | + | − | − | − |
| Hepatomegaly | + | − | − | − | + | + | + | − | − | − | + | + |
| Hernia | + | + | − | + | + | − | + | + | + | + | + | − |
| Joint stiffness | − | + | − | − | − | − | − | − | − | + | + | + |
| Mongolian spot | + | + | − | − | − | − | − | + | − | + | − | − |
| Hypertrichosis | − | − | − | − | − | + | − | − | − | + | + | − |
| Total β-hexosaminidase (μmol/L h) | − | 10,508 | 12,096 | 16,505 | 15,468 | 13,106 | 16,563 | 15,883 | − | − | 16,278 | − |
| α-Mannosidase (μmol/L h) | − | 6252 | 5303 | 4921 | 6609 | 4989 | 5127 | 3650 | − | − | 5644 | − |
| # | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | F | F | M | F | F | F | F | F | F | M | F | M |
| Age at first symptoms (months) | 3 | 3 | 2 | 6 | 2 | 6 | 3 | 3 | 1 | 1 | 1 | 1 |
| Age at diagnosis (months) | 10 | 11 | 6 | 10 | 10 | 9 | 21 | 11 | 25 | 2 | 11 | 2 |
| Current age (months) | 68 | − | 30 | 26 | 20 | − | 30 | − | 41 | 12 | 40 | 9 |
| Parenteral consanguinity | + | + | + | + | + | + | + | + | − | + | + | + |
| Family history | − | − | − | + | + | − | − | − | − | − | − | − |
| IUGR | − | + | − | − | + | − | + | + | − | + | − | + |
| Weight SDS | −2.34 | −3.1 | −2.43 | −3.6 | −4.13 | −3.5 | −2.44 | −1.88 | −2.7 | −2.8 | −2.5 | −3.4 |
| Height SDS | −2.4 | −2.66 | −2.15 | −1.72 | −2.36 | −3.03 | −3.47 | −2.32 | −1.5 | −0.9 | −1.02 | −4.7 |
| Developmental delay | + | + | + | + | + | + | + | + | + | + | + | + |
| Coarse face | + | + | + | + | + | + | + | + | + | + | + | + |
| Corneal clouding | + | + | + | + | + | + | + | + | − | + | − | − |
| Gingival hyperplasia | + | + | + | + | + | + | + | + | + | − | − | + |
| Short neck | − | − | + | + | + | + | + | + | + | + | + | + |
| Chest deformity | − | − | − | + | + | + | − | − | − | − | + | − |
| Cardiac findings | + | + | − | − | − | − | + | + | − | + | + | + |
| Hepatomegaly | − | + | − | + | − | − | − | − | − | − | − | − |
| Hernia | − | + | − | − | − | + | − | + | + | − | + | + |
| Joint stiffness | + | + | + | − | − | + | + | + | + | + | + | + |
| Mongolian spot | − | − | − | − | − | − | − | − | − | − | − | − |
| Hypertrichosis | − | − | − | − | − | + | − | − | − | − | + | + |
| Total β-hexosaminidase (μmol/L h) | 27,994 | 16,830 | 10,230 | 14,919 | − | 29,551 | 13,177 | 15,143 | 15,027 | 16,153 | − | 15,766 |
| α-Mannosidase (μmol/L h) | 1318 | 3955 | − | 1916 | − | 5495 | 3148 | 5782 | 881 | 926 | − | 5382 |
- Abbreviations: F, female; IUGR, intrauterine growth retardation; M, male; SDS, standard deviation score.
The mean follow-up period of the patients was 35.3 ± 20.3 months. Our oldest patient was 6 years and 6 months old. One of the living patients needed noninvasive mechanical ventilation. Sixteen patients had recurrent lower respiratory infections. Twelve patients died due to lung infection and respiratory failure. The mean survival time of these patients was 3.0 ± 1.4 years. The current age of the remaining 12 patients was 36.9 ± 20.6 months.
3.2 Laboratory findings
Urine glycosaminoglycan (GAG) levels were abnormal in seven patients whose urinary GAG levels could be analyzed. Total β-hexosaminidase and α-mannosidase enzyme levels were analyzed in 19 patients and detected to be significantly high in all.
3.3 Radiological findings
In the radiological evaluations of the patients; scaphocephaly (4), J-shaped sella (2), vertebral sclerosis (2), wedge vertebra (4), scoliosis (4), oar-shaped costa (6), developmental dysplasia of the hip (4), bilateral cloaking of the femur (5), bullet shape phalanges (16), talocalcaneal stippling (1), and rickets-like changes (5) were observed. Interestingly, bilateral cloaking of the femur (1 day-2 months), talocalcaneal stippling (13 days), and rickets-like changes (1 day-3 months) were seen only in patients in the first 3 months of life. Of these patients; one was 1 day old, one was 1 month old, two were 2 months old, and one was 3 months old. Whereas, dysostosis multiplex was relatively a late finding (Figures 2-5).
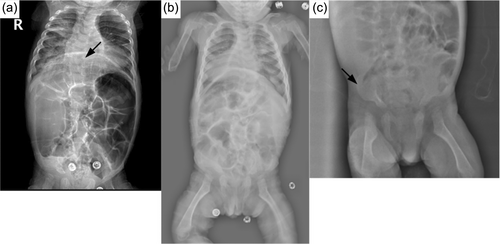
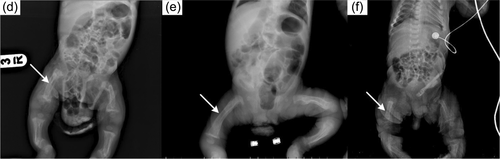
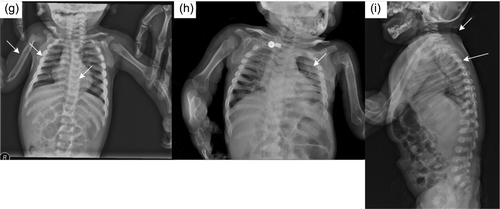
Cardiological evaluations of the patients with echocardiography revealed aortic valve insufficiency in five patients and mitral valve insufficiency in eight patients, mitral valve prolapse in two patients, left ventricular hypertrophy in one patient, intraventricular septum thickening in one patient, and non-compaction cardiomyopathy in one patient.
Detailed ultrasound scans for prenatal diagnosis of our patients, performed by specialist perinatologists were not available.
3.4 Molecular findings
Three patients were diagnosed by elevated lysosomal enzyme levels, while genetic studies could be performed on 21 patients and biallelic pathogenic variants were detected in the GNPTAB gene. The most common pathogenic variant was c.3503-3504delTC (66.6%) (Table 2). Novel pathogenic variants were found in two patients. Parents were heterozygous carriers for all patients, including novel variants. There were two novel variants and one of them was c.2822T>C result in an isoleucine (I) to threonine (T) substitution. The other novel variant was c.235delT (p.Y79Tfs*4) which lead to frameshift and early termination of the protein. In silico programs, Polyphen and SIFT, predicted these variants as likely damaging.
| # | cDNA change | Protein change |
|---|---|---|
| 1 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 2 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 6 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 7 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 8 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 9 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 10 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 11a | Homozygous c.235delT | p.Y79Tfs*4 |
| 12 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 13 | Homozygous c.378-379insA | p.E127Rfs*9 |
| 14 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 15a | Homozygous c.2822T>C | p.I941T |
| 16 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 17 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 18 | Homozygous c.232-234delGTT | p.78delV |
| 19 | Homozygous c.1547A>T | p.D516V |
| 20 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 21 | Compound heterozygous c.3091C>T/c.3503_3504delTC | p.R1031*/p.L1168Qfs*5 |
| 22 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 23 | Homozygous c.3503-3504delTC | p.L1168Qfs*5 |
| 24 | Homozygous c.2991T>G | p.Y997* |
- a Novel pathogenic variants.
4 DISCUSSION
N-acetylglucosaminyl-l-phosphotransferase deficiency results in a rare lysosomal storage disease, ML-II/III. While, ML-II is the severe phenotype which usually results in death in the first decade, ML-III is milder compared to ML-II. For the diagnosis of ML-II, coarse facial appearance and positive family history are critical (Liu et al., 2016). Although some publications consider that patients with ML-II do not have facial dysmorphism as prominent as seen in mucopolysaccharidosis, all of our cases had a prominent coarse facial appearance at first admission (Ceroni et al., 2019). Dogterom et al. (2021) reported dysmorphic facial features (47.4%), developmental delay (24.2%), bone abnormalities (20.0%), growth retardation (12.6%), skull deformities (10.7%), and joint stiffness (10.7%) as common presenting symptoms. The first symptoms noticed in our ML-II patients were coarse facial appearance and hypotonia. Newborn cases had mild coarse facial appearance and gingival hypertrophy was decisive for diagnosis in these cases. In our case series, the mean age of onset of symptoms was 2.7 ± 1.39 months, while the mean age of diagnosis was 9.3 ± 5.7 months. Twenty patients were diagnosed while they were under 1 year of age, and four patients over 1 year of age. In the case series presented by Liu et al. (2016) most patients were diagnosed after 1 year of age. Contrarily, in another study, the age at diagnosis was under 1 year (David-Vizcarra et al., 2010). Awareness of the disease probably affects the age at diagnosis.
Six patients had craniosynostosis, and one of them also had trigonocephaly. Craniosynostosis is previously reported in ML-II patients although it is not clear if it is a consequence of either skeletal or neurological involvements (Cathey et al., 2010; Yang et al., 2020). In a systematic review, 7 out of 70 patients required surgery for craniosynostosis (Dogterom et al., 2021). While none of our patients with craniosynostosis required surgery, it should be considered as important and requires close follow-up.
Growth is impaired in ML-II and in a review 12.6% of the patients were reported to have growth retardation as a presenting symptom (Dogterom et al., 2021). Both height and weight SDS were below −2 for all of our patients. Cathey et al. (2010) reported 12 ML-II patients and all had short stature. So, growth retardation should be considered as a significant sign in these patients.
Cardiac involvement in patients with ML-II can present as the thickening of aortic, mitral, and tricuspid valves and hypertrophic cardiomyopathy (Satoh et al., 1983). In this study; 11 patients had cardiac involvement such as mitral valve insufficiency (33.3%), aortic valve insufficiency (20.8%), mitral valve prolapse (8.3%), left ventricular hypertrophy (4.1%), intraventricular septum thickening (4.1%), and non-compaction cardiomyopathy (4.1%). It is observed that mitral valve was more frequently affected in ML-II in line with the literature. Valve replacement can be performed in patients with heart valve dysfunction (Takanobu, 2018). Dilated cardiomyopathy is an uncommon cardiac feature in ML-II which we did not detect. It has been reported in literature in only a few cases (Carboni et al., 2020).
Hepatomegaly was detected in eight patients. Although patients with hepatosplenomegaly have been reported in the literature, splenomegaly was not detected among our patients (Liu et al., 2016; Otomo et al., 2009). Okada et al. (1985) reported both hepatomegaly and splenomegaly but former is more frequently observed. Of the 24 patients, 15 had umbilical and/or inguinal hernia. Five patients had undergone hernia surgery. Several studies reported hernias as a frequent sign (31.2%–50%) in ML-II patients (Cathey et al., 2010; David-Vizcarra et al., 2010; Liu et al., 2016). Patient “1” admitted with constipation due to ileus. This patient underwent an emergency surgery, and mesocolic hernia was detected. The mesocolic hernia is a very rare cause of hernia with an incidence of 0.2%–0.9% (Chamely & Antao, 2016). The coexistence of mucolipidosis and mesocolic hernia has not been previously reported in the literature. When compared with the literature, the frequency of surgical procedures in case series was less frequent. Dogterom et al. (2021) reported that 32.8% patients needed different kinds of surgical interventions.
In ML-II; kyphoscoliosis, lumbar gibbus, pectus carinatum/excavatum, and restricted joint mobility are other common findings related to skeletal involvement (David-Vizcarra et al., 2010). Radiological evaluation of skeletal involvement is an important diagnostic procedure in mucolipidosis and mucopolysaccharidosis (David-Vizcarra et al., 2010; Spranger, 1988). It usually becomes apparent with age (David-Vizcarra et al., 2010). In our case series; radiological evaluation detected scaphocephaly, J-shaped sella turcica, vertebral sclerosis, wedge vertebra, scoliosis, oar-shaped costa, bilateral cloaking in the femur, bullet-shaped phalanx, talocalcaneal stippling, and developmental dysplasia of hip. Dysostosis multiplex was more prominent in older patients. Rickets-like changes were reported in the neonatal period previously in the literature (Alegra et al., 2013; David-Vizcarra et al., 2010; Lin & Pitukcheewanont, 2012). Interestingly, we observed changes resembling rickets in the radiographs of five patients only between 1 day and 3 months of age. This indicates that these findings can also be observed in the early infancy beyond the neonatal period. Long-bone cloaking is another significant finding seen in the fetal or newborn period which is considered to be highly characteristic of ML-II and disappears in the first year (Lai & Lachman, 2016). In our cases, femoral cloaking was detected in three patients at 2 months, in one patient at 1 month, and in one patient at 1 day. Additionally, similar to the literature, long-bone cloaking was not observed on the radiographs of our older patients. In the case series presented by Lai and Lachman., the stippling in the talocalcaneal region was observed in 86% of fetuses and newborns (Lai & Lachman, 2016). Only one of our patients had talocalcaneal stippling on the X-ray at the age of 13 days. Interestingly, the other patients in the neonatal or early infancy period did not have talocalcaneal stippling. Cloaking, rickets-like changes, and stippling findings were not observed in patients older than 6 months and this suggests that these findings disappear within the first year. In mucolipidosis-II, sacrococcygeal sclerosis, generalized vertebral body sclerosis, and increased vertebral body height were reported as early radiographic findings, which have not been reported in other mucolipidosis (Lai & Lachman, 2016). Two of our patients had vertebral sclerosis. All these radiological features point out that the early radiological findings of ML-II are different from dysostosis multiplex.
Prenatal diagnosis and genetic counseling for hereditary diseases are available once an index case is defined in a family. For this reason, it is critical to define clinical features suggesting specific diagnoses in the antenatal period to facilitate prenatal diagnosis. In mucolipidosis, oligohydramnios, and IUGR may be observed in the intrauterine period, although nonspecific (Cathey et al., 2010; Chen et al., 2010; Costain et al., 2018; Liu et al., 2016). In our case series, 10 patients had a history of IUGR. Apart from this, nonimmune hydrops fetalis indicates lysosomal storage diseases including mucolipidosis, which was not seen in our patients. There are cases in the literature which ultrasonography detected femoral cloaking, short femur, curved bone, echogenic cardiac foci, and transient maxillary alveolar defect in the intrauterine period (Cathey et al., 2010; Chen et al., 2010; Costain et al., 2018; Liu et al., 2016). Although these findings are not specific, they can still be red flags. Patient 24 who was in the neonatal intensive care unit due to respiratory distress was consulted due to the short femur detected in the intrauterine period. Later, ML-II was suspected after observing the presence of cloaking and rickets-like changes in the radiographs. Therefore, prenatal radiological findings and detailed prenatal history may facilitate early diagnosis.
Several pulmonary complications have been described in ML-II patients such as thickening of the respiratory mucosa, lipid granulomata, restrictive lung disease due to a small rib cage, and pulmonary hemorrhage (Ishak et al., 2012). Therefore, most patients may have recurrent respiratory tract infections and respiratory failure. In these patients, nasal continuous positive airway pressure (CPAP) treatment has been shown to be beneficial since it reduces the increased airway resistance, and CPAP support is recommended immediately in patients with respiratory failure symptoms (Edmiston et al., 2019; Sheikh et al., 1998). In our study, 66.6% patients had frequently recurrent lower respiratory infections. Two patients required invasive mechanical ventilation while receiving pneumonia treatment. Subsequently, both of these patients needed tracheostomy and one of them also needed CPAP.
ML-II usually results in death in the first decade. Recurrent lung infections and respiratory failure are major causes of death (Spranger et al., 2012). Twelve of our patients died during their follow-up due to severe lung infection and respiratory failure. The age at death ranged from 7 months to 5 years. The mean survival time was 3.01 ± 1.4 years. In the case series of (David-Vizcarra et al., 2010) the median survival time was reported to be 27 months.
In ML-II patients, the most common pathogenic variant in the GNPTAB gene was c.3503-3504delTc deletion (Cathey et al., 2010; Coutinho et al., 2011; Liu et al., 2016; Plante et al., 2008; Tappino et al., 2009). Likewise, the most common (66.6%) pathogenic variant in our cases was c.3503-3504delTc deletion. In two patients, novel pathogenic variants were detected. No significant phenotypic difference was observed among these patients and there was no clear genotype–phenotype correlation.
ML-II does not yet have curative treatment. Nutritional support, physiotherapy, respiratory physiotherapy, CPAP, and bisphosphonate treatment are supportive treatments that improve the quality of life. Studies on enzyme replacement therapy are still insufficient. One of the biggest challenges in this treatment is that there are multiple mannose 6-phosphorylated lysosomal enzymes (Khan & Tomatsu, 2020). Recent studies have shown that bone marrow transplantation is not effective due to its side effects, such as the risk of infection (Lund et al., 2014). According to a study conducted on mice, related to gene therapy, inhibition of IL-6 production can prevent bone loss by showing that there is an increase in bone mineral density and a decrease in IL-6 expression in treated mice (Ko et al., 2016).
5 CONCLUSION
Knowing the natural course of all clinical and radiological features of ML-II is very important in terms of early diagnosis, as it provides the opportunity to provide genetic counseling to the family and gain a better insight about the disease course. Therefore, we presented our case series to contribute to a better definition of clinical and radiological features and to emphasize the clues that guide to the diagnosis. The importance of early diagnosis will increase even more when clinical studies for new therapeutic options become available.
AUTHOR CONTRIBUTIONS
Ezgi Burgaç: Collecting the data, drafting and writing the manuscript. İrem Kaplan: Formal analysis. Burcu Köseci: Visualization. Esra Kara: Visualization. Deniz Kor: Conceptualization; supervision. Fatma Derya Bulut: Conceptualization; supervision. Anıl Atmış: Visualization. Ferhatcan Pişkin: Processing images. Sevcan Tuğ Bozdoğan: Genetic analysis. Gizem Urel Demir: Genetic analysis. Faruk İncecik: Data curation. Neslihan Önenli Mungan: Writing-review and editing, supervision. All authors approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank the patients for their generous participation in this study.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare in this study.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.