Clinical phenotypes of individuals with Chung–Jansen syndrome across age groups
Abstract
Pathogenic variants in pleckstrin homology domain interacting protein (PHIP) are associated with Chung–Jansen syndrome characterized by developmental delay, intellectual disability, behavioral challenges, hypotonia, obesity, and dysmorphic features. We report phenotypes and genotypes of 47 individuals with likely pathogenic/pathogenic PHIP variants. Variants were de novo in 61.7%, unknown inheritance in 29.8%, and inherited in 8.5%. The median age of the individuals was 10.9 years, approximately equally divided by sex. Individuals in this cohort frequently had a history of developmental delay (85.1%), attention-deficit/hyperactivity disorder (51.1%), anxiety (46.8%), depression (27.7%), and sleep difficulties (42.6%). Depression was significantly higher in the older age group (>12 years old). Most individuals had moderately low adaptive functioning based on the Vineland-3 (mean = 76.8, standard deviation = 12.0). Overall, 55.8% of individuals were obese/overweight. The percentage of obese individuals was greater in the older age group (>12 years old) and evolves over time. Other common symptoms were hypotonia (78.7%), constipation (48.9%), visual problems (66%), and cryptorchidism (39.1% of males). Our findings provide additional natural history data for Chung–Jansen syndrome and provide opportunities for early intervention of healthy eating habits and awareness of developing mood and behavioral challenges over the life course.
1 INTRODUCTION
Pathogenic variants in pleckstrin homology domain interacting protein (PHIP) are associated with Chung–Jansen syndrome (CHUJANS) (OMIM # 617991) characterized by developmental delay, intellectual disability, anxiety, hypotonia, poor balance, obesity, and dysmorphic features. The majority of variants are de novo although inherited variants are observed. The mutation spectrum includes nonsense, frameshift, deletions, and missense, and the mechanism of action is loss of function leading to haploinsufficiency or reduced function of the PHIP gene product.
PHIP1 contains four protein regions: WD40 repeats, PH binding region domain, two bromodomains, and a nuclear localization signal (Craddock et al., 2019). The PHIP1 or DCAF14 is a DDB1-CUL4-associated factor (DCAF) protein family member that acts as substrate receptors for ubiquitin E3 ligases through the CUL4–DDB1 complex, which regulates cell proliferation, survival, DNA repair, and genomic integrity (Jin et al., 2006; Lee & Zhou, 2007). In addition, PHIP binds the pleckstrin homology domain of insulin receptor substrate-1, modulates insulin signaling (Farhang-Fallah et al., 2000), plays a role in insulin receptor-mediated mitogenic and metabolic signal transduction, and regulates glucose transporter translocation in skeletal muscle cells (Farhang-Fallah et al., 2002) and pancreatic beta cell growth and survival (Podcheko et al., 2007). Moreover, nuclear PHIP enhances the transcription of pro-opiomelanocortin (POMC) that suppresses appetite and may provide mechanistic insight into the association with obesity (Marenne et al., 2020).
To expand our understanding of the clinical features associated with CHUJANS, we have expanded our cohort to 47 individuals with pathogenic/likely pathogenic PHIP variants (Craddock et al., 2019).
2 MATERIALS AND METHODS
This study was approved by the Columbia University Institutional Review Board, and online informed consent was obtained from all participants or their guardians. The study recruited individuals with PHIP variants classified as pathogenic or likely pathogenic according to the American College of Medical Genetics (ACMG) classification guideline (Richards et al., 2015). All participants had previously undergone whole exome sequencing, whole genome sequencing, or panel gene testing and many also had chromosome microarray. Clinical genetic test reports were submitted and reviewed by a geneticist, and any dual genetic diagnoses were excluded. Clinical history and physical examination data were collected via patient report during a medical interview by phone with a physician or genetic counselor, along with verification by medical record review when possible. In addition, the Vineland Adaptive Behavior Scales Third Edition (Vineland-3) comprehensive parent/caregiver form (Sparrow et al., 2016) was completed by the guardians or self-report using the online Pearson's Q-global™ platform to assess adaptive function.
Body mass index (BMI; weight in kg/height in m2) was calculated in individuals ≥2 years old. For those age 2–20 years old, overweight was defined as BMI from the 85th percentile (z-score = 1.0) to less than the 95th percentile (z-score = 1.7), obesity for BMI ≥95th percentile, and severe obesity for BMI ≥120% of the 95th percentile (z-score ≥2.0). For those ≥20 years old, overweight was defined as BMI of 25–30 kg/m2, obese for BMI ≥30 kg/m2, and severe obesity of BMI ≥40 kg/m2. For those <2 years old, overweight was defined as weight-for-length >97 percentile and obesity for weight-for-length >99 percentile.
2.1 Statistical analysis
The data were analyzed using IBM SPSS (version 28). Categorical variables are reported as percentage (%). Continuous variables with normal distribution are reported as mean (M) with standard deviation (SD); non-normal distribution variables are reported as median with interquartile range (IQR). The frequencies of categorical variables were compared using the chi-square test. Cochran–Mantel–Haenszel tests were used for stratified analysis by age group. Student's t-test was used to compare means of two groups of continuous normally distributed variables. A Pearson's correlation coefficient was computed to assess the relationship between the BMI z-score and age. P-value is adjusted for multiple comparisons using the Bonferroni correction. A value of p ≤ 0.05 was considered significant.
3 RESULTS
Forty-seven individuals were enrolled in the study. Individuals were approximately equally divided by sex (female 51.1%). The median age of the individuals was 10.9 (IQR = 11.5) years, with ages ranging from 5 months to 43.7 years old (Table 1). The majority of the individuals were minors <18 years old (76.6%).
| Clinical characteristics | Study cohort |
|---|---|
| Age, median (IQR) | 10.9 (11.5) |
| Sex (n = 47), (%) | |
|
48.9 |
|
51.1 |
| Weight BMI (n = 43), (%) | |
|
39.5 |
|
16.3 |
|
41.9 |
|
2.3 |
| Visual problems (n = 47), (%) | |
|
66.0 |
|
23.4 |
|
17.0 |
|
17.0 |
|
12.8 |
|
10.6 |
|
4.3 |
|
4.3 |
|
4.3 |
|
2.1 |
|
2.1 |
| ENT problems (n = 47), (%) | |
|
44.7 |
|
21.3 |
|
31.9 |
|
12.8 |
| Neurological problems (n = 47), (%) | |
|
78.7 |
|
29.8 |
|
21.3 |
|
10.6 |
|
6.4 |
|
6.4 |
|
4.3 |
| Gastrointestinal problems (n = 47), (%) | |
|
48.9 |
|
23.4 |
|
2.1 |
|
2.1 |
|
2.1 |
| Respiratory problems (n = 47), (%) | |
|
27.7 |
|
4.3 |
| Cardiovascular problems (n = 47), (%) | |
|
8.5 |
|
4.3 |
| Endocrine problems (n = 47), (%) | |
|
8.3 |
|
6.4 |
|
4.3 |
|
2.1 |
|
2.1 |
|
2.1 |
| Urogenital problems (n = 47), (%) | |
|
29.8 |
|
39.1 |
|
8.5 |
|
4.3 |
|
4.3 |
| Dermatologic problems (n = 47), (%) | |
|
21.3 |
|
4.3 |
| Orthopedics problems (n = 47), (%) | |
|
25.5 |
|
14.9 |
|
14.9 |
|
4.3 |
|
2.1 |
| Developmental, mood, and behavioral challenges (n = 47), (%) | |
|
85.1 |
|
51.1 |
|
29.8 |
|
46.8 |
|
27.7 |
|
6.4 |
|
42.6 |
- Abbreviations: ENT, ear, nose, and throat; IQR, interquartile range; PE, pressure equalization.
3.1 Genetic variants
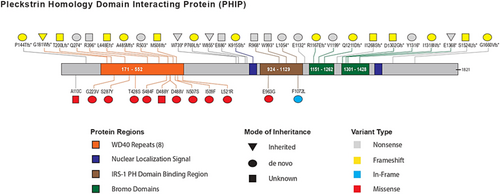
All participants had likely pathogenic or pathogenic PHIP variants (12 missense variants, 13 nonsense variants, 15 frameshift variants, 4 splice variants, 1 inframe variant, and 2 multigene deletions). The two multigene deletions include the IRAK1BP1 and PHIP genes. Variants were demonstrated to be de novo in 29 cases (61.7%), inherited in 4 cases (8.5%), and of unknown inheritance due to lack of parental testing in 14 cases (29.8%). The inherited variants were three nonsense variants (including one from a mosaic parent with 10% mosaicism) and one frameshift variant (Table 2). Two parents reported learning difficulties and one parent had anxiety. Almost all missense variants are in the WD40 repeat regions (Figure 1).
| Chromosome change (Hg38) | HGVS DNA reference (NM_017934) | HGVS protein reference | Variant type | ACMG/AMP 2015 classification | Inheritance | ClinVar accession | |
|---|---|---|---|---|---|---|---|
| 1 | g.79077920G>T | c.41-7C>A | Missense | LP (PS2, PM2, PP3) | De novo | ||
| 2 | g.79060680G>A | c.328C>T | p.Arg110Cys | Missense | LP (PM1, PM2, PP3) | Unknown | VCV000975951.8 |
| 3 | g. 79060489dup | c.428dupC | p.Pro144ThrfsTer19 | Frameshift | LP (PVS1, PS2, PM2) | De novo | VCV002446034.1 |
| 4 | g. 79060473C>A | c.439+5G>T | Splice site | LP (PS2, PM2, PP3) | De novo | ||
| 5 | g. 79042903dup | c.540dup | p.Gly181TrpfsTer12 | Frameshift | LP (PVS1, PM2) | Paternal | VCV000627523.2 |
| 6a | g.79042844_79042845delinsA | c.598_599delACinsT | p.Thr200LeufsTer8 | Frameshift | LP (PVS1, PM2) | Unknown (not paternal) | VCV000524089.4 |
| 7 | g. 79026097C>A | c.668G>T | p.Gly223Val | Missense | LP (PS2, PM2, PP3) | De novo | VCV000965295.6 |
| 8 | g. 79025945G>A | c.820C>T | p.Gln274Ter | Nonsense | P (PVS1, PS2, PM2) | De novo | |
| 9a | g. 79025582G>T | c.860C>A | p.Ser287Tyr | Missense | LP (PS2, PM2, PP3) | De novo | VCV000426892.6 |
| 10 | g. 79016593G>A | c.1186C>T | p.Arg396Ter | Nonsense | LP (PVS1, PM2) | Unknown | VCV001686057.3 |
| 11 | g. 79015736G>C | c.1283C>G | p.Thr428Ser | Missense | LP (PS2, PM2, PP3) | De novo | |
| 12 | g. 79015676_79015677del | c.1342_1343del | p.Leu448GlufsTer18 | Frameshift | LP (PVS1, PM2) | Unknown | VCV000424321.2 |
| 13 | g.79015155G>A | c.1451C>T | p.Ser484Phe | Missense | LP (PS2, PM2, PP3) | De novo | |
| 14 | g. 79015148_79015154del | c.1452_1458del | p.Ala485MetfsTer5 | Frameshift | LP (PVS1, PM2) | De novo | |
| 15 | g. 79015144C>A | c.1462G>T | p.Asp488Tyr | Missense | LP (PM2, PP3, PM5) | Unknown | VCV001299330.1 |
| 16 | g. 79015143T>A | c.1463A>T | p.Asp488Val | Missense | LP (PS2, PM2, PP3) | De novo | VCV001695372.1 |
| 17 | g. 79015099G>A | c.1507C>T | p.Arg503Ter | Nonsense | P (PVS1, PM1, PM2) | De novo | VCV000987322.2 |
| 18 | g. 79015086T>C | c.1520A>G | p.Asn507Ser | Missense | LP (PS2, PM2, PP3) | De novo | |
| 19 | g. 79015083dup | c.1523dup | p.Met508IlefsTer3 | Frameshift | LP (PVS1, PM2) | Unknown | |
| 20 | g.79003858T>A | c.1525A>T | p.Ile509Phe | Missense | LP (PS2, PM2, PP3) | De novo | |
| 21a | g.79003821T>C | c.1562A>G | p.Lys521Arg | Missense | LP (PS2, PM2, PP3) | De novo | VCV000627526.5 |
| 22 | g.79002126T>G | c.1654-2A>C | Splice site | P (PVS1, PS2, PM2) | De novo | ||
| 23 | g.78990971C>T | c.2216G>A | p.Trp739Ter | Nonsense | LP (PVS1, PM2) | Paternal mosaic | |
| 24 | g.78990878_78990881del | c.2306_2309del | p.Pro769LeufsTer43 | Frameshift | P (PVS1, PS2, PM2) | De novo | |
| 25 | g.78990867del | c.2319+1del | Splice site | P (PVS1, PS2, PM2) | De novo | VCV000684552.7 | |
| 26 | g.78983091C>T | c.2564G>A | p.Trp855Ter | Nonsense | LP (PVS1, PM2) | Maternal | VCV001324892.2 |
| 27 | g.78982999C>A | c.2656G>T | p.Glu886Ter | Nonsense | LP (PVS1, PM2) | Unknown | VCV001722952.2 |
| 28a | g.78982908_78982911del | c.2744_2747del | p.Lys915SerfsTer15 | Frameshift | P (PVS1, PS2, PM2) | De novo | VCV000627525.2 |
| 29 | g.78978593T>C | c.2888A>G | p.Glu963Gly | Missense | LP (PS2, PM2, PP3) | De novo | VCV001695371.1 |
| 30 | g.78970890T>C | c.2890-2A>G | Splice site | LP (PS2, PM2, PP3) | De novo | ||
| 31 | g.78970876G>A | c.2902C>T | p.Arg968Ter | Nonsense | LP (PVS1, PM2) | Unknown | VCV000523846.8 |
| 32 | g.78970800C>T | c.2978G>A | p.Trp993Ter | Nonsense | LP (PVS1, PM2) | Unknown | |
| 33a | g.78969879del | c.3161del | p.Leu1054Ter | Nonsense | P (PVS1, PS2, PM2) | De novo | VCV000627527.2 |
| 34 | g.78966035_78966046del | c.3216_3227del | p.Phe1072_Ile1076delinsLeu | Inframe deletion | LP (PS2, PM2) | De novo | |
| 35 | g.78963238C>A | c.3394G>T | p.Glu1132Ter | Nonsense | P (PVS1, PS2, PM2) | De novo | |
| 36 | g.78963133del | c.3499del | p.Arg1167GlufsTer6 | Frameshift | P (PVS1, PS2, PM2) | De novo | |
| 37a | g.78961751del | c.3595delG | p.Val1199Ter | Nonsense | P (PVS1, PS2, PM2) | De novo | VCV000627522.2 |
| 38 | g.78961712_78961715del | c.3628_3631del | p.Gln1211AspfsTer13 | Frameshift | P (PVS1, PS2, PM2) | De novo | |
| 39 | g.78955660_78955664del | c.3801_3805del | p.Ile1268SerfsTer4 | Frameshift | LP (PVS1, PM2) | Unknown | VCV001700481.2 |
| 40 | g.78955233dup | c.3902dup | p.Asp1302GlyfsTer5 | Frameshift | LP (PVS1, PM2) | Unknown | |
| 41 | g.78954920dup | c.3947dup | p.Tyr1316Ter | Nonsense | P (PVS1, PS2, PM2) | De novo | VCV000817578.3 |
| 42 | g.78954915dup | c.3952dup | p.Ile1318AsnfsTer9 | Frameshift | P (PVS1, PS2, PM2) | De novo | |
| 43 | g.78947727C>A | c.4102G>T | p.Glu1368Ter | Nonsense | LP (PVS1, PM2) | Maternal | |
| 44a | g.78946061del | c.4570del | p.Ser1524LeufsTer22 | Frameshift | LP (PVS1, PM2) | Unknown | VCV000627524.2 |
| 45 | g.78941180del | c.4979del | p.Gly1660ValfsTer15 | Frameshift | LP (PS2, PM2, PP3) | De novo | |
| 46 | 6q14.1(979098359_79910342)x1 | Unknown | |||||
| 47 | 6q14.1(79576089_79665445)x1 | Unknown |
- a Individuals previously reported in Craddock et al. (2019).
3.2 Clinical characteristics
3.2.1 Developmental and behavioral issues in individuals with PHIP variants
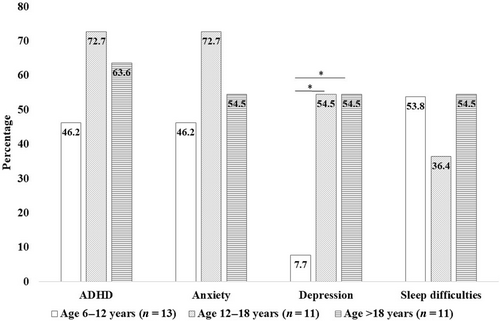
Individuals in this cohort frequently had a history of developmental delay (85.1%) and autism spectrum disorder (29.8%). Behavioral conditions included attention-deficit/hyperactivity disorder (ADHD) (51.1%), anxiety (46.8%), and depression (27.7%). Sleep difficulties were frequently noted (42.6%). The percentage of individuals with depression was significantly greater in the older age group (>12 years old) (adjusted p < 0.05). However, the frequency of ADHD, anxiety, and sleep difficulties were not different by age (Figure 2).
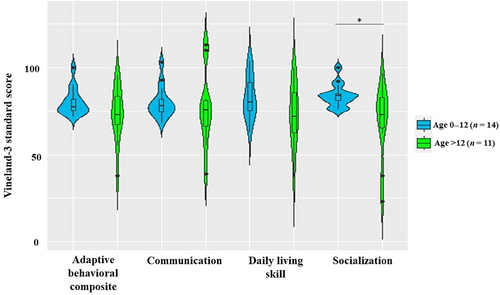
Many but not all individuals had moderately low adaptive functioning based upon the adaptive behavior composite (ABC) standard score in the Vineland adaptive behavioral scale (M = 76.8, SD = 12.0). All subdomains are approximately equally affected: communication (M = 78.8, SD = 15.6), daily living skills (M = 78.7, SD = 15.8), socialization (M = 77.6, SD = 16.5), and motor skills (M = 79.3, SD = 16.7). The ABC standard score ranged from 38 in a 23.3-year-old woman to 100 in a 4-year-old woman, both with missense variants. Individuals ≥12-year-old had significantly lower scores (M = 69.4, SD = 21.6) compared to the younger age group (age <12 years old) (M = 84.0, SD = 6.6) in the socialization subdomain (t = 2.41, p < 0.05) (Figure 3).
3.2.2 Weight of individuals with PHIP variants
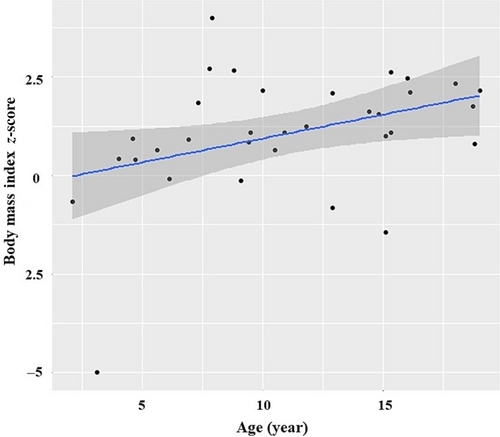
We had data for weight and height in 43 individuals. The mean height z-score was −0.3, SD = 1.0. For individuals <20 years old, obesity was observed in 37.8% (14/37) and overweight in 13.5% (5/37), with a mean BMI z-score of 1.1 (SD = 1.6) and a range of −4.7 to 4.0. For individuals >20 years old, obesity was observed in 50% (3/6), and overweight in 50% (3/6), with a mean BMI in adults of 36.6 kg/m2 (SD = 11.1). Overall, 39.5% (17/43) of individuals were obese, 16.3% (7/43) were overweight, and 2.3% (1/43) were underweight. Severe obesity was observed at 25.6% (11/43). The percentage of obese/overweight individuals was greater in the older age group (>12 years old) (X2 (3, N = 43) = 18.36, p < 0.001). A positive correlation was observed between age and BMI z-score, r(33) = 0.38, p < 0.05 (Figure 4).
Obesity/overweight was found to be positively associated with depression (X2 [1, N = 43] = 4.1, p < 0.05). When stratifying the association between obesity/overweight and depression by age group, there was a positive association in the adolescent group (Supplement Table 1). No association between obesity/overweight and anxiety was found (X2 [1, N = 43] = 3.1, p > 0.05).
3.2.3 Other clinical symptoms in individuals with PHIP variants
Neurological symptoms were common and included hypotonia (78.7%), excessively clumsy or uncoordinated (29.8%), and movement abnormalities (21.3%) including tremors with an onset at 4 (IQR = 10.5) years old when present. Visual problems were reported in 66% and included myopia (23.4%), hyperopia (17%), and strabismus (17%). Gastrointestinal problems were common and included constipation in 48.9% and gastroesophageal reflux disease in 23.4%. Congenital kidney malformations were found in four individuals (two with horseshoe kidneys and two with unilateral kidney). Cryptorchidism was reported in 39.1% of males and five males had orchiopexy repair. History of otitis media was reported in 44.7% and a frequency more than five times in life in 21.3%; treatment with tube placement occurred in 21.3%. Hearing problems were reported in 12.8%, with an average age of onset of 2.1 (SD = 0.6) years old. Most of the hearing problems were conductive hearing loss (7/8). Orthopedic problems included scoliosis in 25.5%, clinodactyly of the fifth finger in 14.9%, and syndactyly of the toes in 14.9%. Two individuals had a ventricular septal defect. One individual had Type 2 diabetes mellitus.
A positive association between constipation and ADHD was shown (X2 [1, N = 47] = 6.0, p < 0.05); individuals with constipation were more frequently diagnosed with ADHD. No association was observed between constipation and developmental delay (X2 [1, N = 47] = 0.98, p > 0.05).
3.2.4 Perinatal history of individuals with PHIP variants
Most individuals were born full term (gestational age M = 38.6 weeks, SD = 2.1) with normal birth weight and length when adjusted for gestational age. Preterm delivery was reported in 17%, with the earliest gestational age of 33 weeks. The most common pregnancy complications were preeclampsia (10.6%) and polyhydramnios (8.5%). The most common neonatal problems were hypotonia (48.9%), feeding difficulty (44.7%), and hyperbilirubinemia (38.3%) (Table 3).
| Perinatal history | Study cohort |
|---|---|
| Pregnancy complication (n = 47), (%) | |
|
10.6 |
|
8.5 |
|
6.4 |
|
4.3 |
|
4.3 |
|
2.1 |
| Gestational age (day) (n = 43), mean ± SD | 38.6 ± 2.1 |
| Birth weight (z-score) (n = 39), mean ± SD | −0.1 ± 1.2 |
| Birth length (z-score) (n = 32), mean ± SD | 0.7 ± 1.0 |
| Birth head circumference (z-score) (n = 10), mean ± SD | −0.2 ± 0.6 |
| Apgar score (n = 12), mean ± SD | 1 min: 8.2 ± 1.8, 5 min: 9.2 ± 0.8 |
| Hospital stay (day) (n = 41), median (IQR) | Median 2.8 (3) |
| NICU stay (n = 47), (%) | 10.6 |
| Perinatal complications (n = 47), (%) | |
|
17.0 |
|
10.6 |
|
6.4 |
|
6.4 |
| Neonatal problems (n = 47), (%) | |
|
48.9 |
|
44.7 |
|
38.3 |
|
17.0 |
|
14.9 |
|
4.3 |
- Abbreviations: IQR, interquartile range.
No significant differences were observed in clinical features across mutation locations or when comparing individuals with loss of function and missense variants (Supplement Table 2).
4 DISCUSSION
This series reports on 47 individuals with PHIP variants. The most common clinical symptoms were developmental delay, behavioral challenges, hypotonia, coordination disorder, visual issues, obesity, and constipation.
Compared to the previous studies (Table 4), we found comparable results with developmental delay in 85% (Jansen et al., 2018; Kampmeier et al., 2022). Our cohort had no report of developmental regression. The adaptive function of the individuals was at a borderline (moderately low) level, which is different from most other monogenetic neurodevelopmental disorders that are associated with moderate to severe intellectual disability (Hanly et al., 2021; O'Brien et al., 2019). This likely explains the higher frequency of inherited variants compared with other neurodevelopmental disorders (Brunet et al., 2021).
| Clinical | Jansen et al. (N = 23) | Kampmeier et al. (N = 23) | This cohort (N = 47) |
|---|---|---|---|
| Age, median (range) | 14 (5–52) | 13 (5–54) | 10.9 (0.4–43.7) |
| Sex, male (%) | 47.8 | 56.5 | 48.9 |
| Developmental delay/Intellectual disability (%) | 82.6 | 100 | 85.1 |
| Behavioral problems and mood disorders (%) | 78 | 87 | 70.2 |
| Sleeping difficulties (%) | 18 | 26.1 | 42.6 |
| Obesity/overweight (%) | 74 | 69.6 | 55.8 |
| Hypotonia (%) | 26 | 34.8 | 78.7 |
| Clumsiness/uncoordinated (%) | — | 30.4 | 29.8 |
| Seizure (%) | 4.3 | 17.4 | 6.4 |
| Feeding difficulty in neonatal period (%) | 26.1 | 18 | 44.7 |
| Neonatal hyperbilirubinemia (%) | — | 21.7 | 38.3 |
| Constipation (%) | 8 | 34.8 | 48.9 |
| Visual problems (%) | 65 | 47.8 | 66.0 |
| Undescended testis (%) | 27.3 | 26.1 | 39.1 |
| Clinodactyly (%) | 64 | 30.4 | 14.9 |
| Syndactyly (%) | 30 | 26.1 | 14.9 |
Our study reported behavioral problems and mood disorders in 70%, which is slightly lower than the previous studies of Jansen et al. and Kampmeier et al. and may be explained by the younger age of our cohort. We showed that behavioral disorders are diagnosed as individuals age. We observed a higher frequency of depression and lower socialization subdomain scores on the Vineland-3 in the older age group. Studies (Hassiotis et al., 2008; Nouwens et al., 2017) showed that children with mild-to-borderline intellectual disabilities had lower self-esteem, higher anxiety/depressed mood, and more personal and social problems. Social abilities such as perspective-taking, interpretation of social situations, and problem-solving are particularly impaired (Alesi et al., 2015). In addition, with increasing age, social skills become more challenging, and the gap between cognitive and emotional abilities and social demands increases, augmenting social difficulties. Moreover, the increase in obesity may exacerbate the mood (Mansur et al., 2015) and social challenges (Albano et al., 2019). We found a positive association between obesity/overweight and depression in the adolescent group in this cohort. Focused longitudinal studies are needed to assess the evolving behavioral challenges and how these evolve and how they are related to body weight regulation.
Obesity in this cohort was reported in adults 50% and in children 38%, which are higher than the general US population of 41.9% in adults and 19.7% in children (Stierman et al., 2021). We report a lower frequency of obesity/overweight compared to previous studies, and this may be explained by the younger age of our cohort and the increase in obesity as individuals age. In our series, there was no obesity in an individual under the age of six, and this age group was 26% of the cohort. We started to observe obesity/overweight at the age of seven, and the frequency of obesity/overweight increases with age. Kampmeier et al. (2022) found that the incidence of obesity/overweight rose sharply during puberty. However, Kampmeier et al. also found that some individuals as young as 5 years old were already obese/overweight. Marenne et al. (2020) found that PHIP variants repressed POMC transcription and this may contribute to the development of obesity due to increased caloric intake. POMC knockout mice exhibit a later onset of obesity, due to greater food intake (Yaswen et al., 1999). PHIP individuals also are hypotonic and may have decreased caloric requirements. Combined with developmental delay and psychological issues such as ADHD and depression, this may exacerbate feeding behaviors and make it difficult for behavioral modification due to differences in cognition, impulsivity, and self-regulation. Additional studies focused on the cause of weight gain, along with ingestive behavior and energy requirements are needed to develop an effective treatment strategy for obesity in this condition. Setmelanotide (Markham, 2021), a melanocortin-4 (MC4) receptor agonist developed for the treatment of obesity on POMC, proprotein convertase subtilisin/kexin Type 1 (PCSK1), or leptin receptor deficiency may be effective. Close caretaker supervision in controlling food intake and portion size and behavioral modification starting at a young age in individuals with a PHIP variant may be helpful in the prevention of future obesity.
We identified more frequent sleeping difficulties and constipation problems than previous reports, and this might be explained by our data collection method. Our study collected data through the medical interview with specific probe questions. We asked every parent and individual about each problem, which may more systematically assess issues that some parents may not raise in a clinical setting. In contrast, we reported a lower frequency of clinodactyly or syndactyly, possibly due to subtle differences not important to parents.
This cohort noted constipation in 49% of individuals. The onset of constipation is as early as 5 months old, and this may be due to hypotonia, an immature connection between the central nervous system and the enteric nervous system (McKeown et al., 2013), or from developmental delay or psychological problems such as ADHD that may affect toilet training or behavioral modification in the later age (Ho & How, 2020). However, in this cohort, we only found an association between constipation and ADHD.
We had families with inherited nonsense and frameshift variants. The phenotype of the parents was mild which may be explained by variable expressivity (Kingdom & Wright, 2022). Variable penetrance was also reported by Marenne et al. (2020) with no obesity or developmental delay in family members carrying the PHIP variants.
5 CONCLUSION
The most common clinical characteristics of individuals with PHIP pathogenic variants are neurobehavioral with developmental delay, ADHD, anxiety, hypotonia, visual problems, and obesity/overweight. The adaptive function of the individuals is borderline (moderately low) level. Congenital anomalies are minor and include cryptorchidism, syndactyly, and clinodactyly. The frequency of obesity/overweight increases with age and early initiation of healthy eating habits may be beneficial. Behavioral health issues also increase with age and may be related to the lower score on the socialization domain of the Vineland and self-awareness of differences and may benefit from support to integrate individuals into their communities.
AUTHOR CONTRIBUTIONS
Khemika K. Sudnawa made substantial contributions to the conception and design, acquisition of data, analysis, and interpretation of data, involved in drafting the manuscript, revising it critically for important intellectual content, and giving final approval of the version to be published. Khemika K. Sudnawa agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Sean Calamia made substantial contributions to the conception and design, acquisition of data, revised it critically for important intellectual content, and gave final approval of the version to be published. Sean Calamia agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Alexa Geltzeiler made substantial contributions to conception and design, acquisition of data, revised it critically for important intellectual content, and gave final approval of the version to be published. Alexa Geltzeiler agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Wendy K. Chung made substantial contributions to the conception and design, acquisition of data, and interpretation of data, involved in drafting the manuscript, revised it critically for important intellectual content, and gave final approval of the version to be published. Wendy K. Chung agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGMENT
We thank the individuals and their families who participated in this study.
FUNDING INFORMATION
The work was funded by NIH grant NIDDK 52431.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




