Diagnostic yield of chromosomal microarray in the largest Latino clinical cohort
Abstract
Copy number variants (CNVs) remain a major etiological cause of neurodevelopmental delay and congenital malformations. Chromosomal microarray analysis (CMA) represents the gold standard for CNVs molecular characterization. We applied CMA throughout the patient's clinical diagnostic workup, as the patient's medical provider requested. We collected CMA results of 3380 patients enrolled for 5 years (2016–2021). We found 830 CNVs in 719 patients with potential clinical significance, that is, (i) pathogenic, (ii) likely pathogenic, and (iii) variants of uncertain significance (VUS), from which 10.6% (predominantly involving chromosomes 15 and 22) were most likely the final cause underpinning the patients' clinical phenotype. For those associated with neurodevelopmental phenotypes, the rate of pathogenic or likely pathogenic findings among the patients with CNVs was 60.75%. When considering epileptic phenotypes, it was 59%. Interestingly, our protocol identified two gains harbored in 17q21.31 and 9q34.3, internationally classified initially as VUS. However, because of their high frequency, we propose that these two VUS be reclassified as likely benign in this widely heterogeneous phenotypic population. These results support the diagnostic yield efficiency of CMA in characterizing CNVs to define the final molecular cause of genetic diseases in this cohort of Colombian patients, the most significant sample of patients from a Latino population, and define new benign polymorphic CNVs.
1 INTRODUCTION
Pathogenic copy number variants (CNVs) represent a significant cause of neurodevelopmental delay, intellectual disability, and congenital anomalies (Karamperis et al., 2020; Miller et al., 2010), underpinning very well-known and new syndromic phenotypes (Shaikh, 2017).
The ACMG has recently recommended the use of whole exome or whole genome sequencing (WES and WGS) as a first-level test for patients with congenital anomalies-malignancies or intellectual disability (Stark et al., 2016), but chromosomal microarrays analysis (CMA) continues to be described as a valuable tool for the diagnosis of phenotypes associated with CNVs, especially outside of the Unite States (Kligler et al., 2021).
Different molecular and cytogenetic assays, such as karyotypes, fluorescence in situ hybridization (FISH), multiplex ligation-dependent probe amplification, and next-generation sequencing (NGS), allow CNVs identification and characterization. Still, the (CMA) improved the CNVs yield (Miller et al., 2010; Riggs et al., 2020) tremendously and is now widely accepted as the standard gold method for the identification and clinical diagnosis of chromosome aneuploidies, microdeletions, and microduplications (Russo et al., 2014).
There are two main methods to perform CMAs: The CGH-based arrays CMA compares two DNA samples (reference and test) labeled with fluorophores by hybridizing the samples with synthetic DNA targets fixed in microchips. The quantification and comparison of the fluorescence level of each of the samples detect losses or gains in DNA sequences harbored in specific regions of the genome (Bejjani & Shaffer, 2006; Korf & Sathienkijkanchai, 2009).
On the other side, single nucleotide polymorphisms (SNP) Microarray Analysis uses high-density oligonucleotide-based arrays, in which only the DNA from the patient is labeled and hybridized to the SNP array. Using in silico tools, CNVs can be identified comparing the absolute fluorescence probe intensities of the patient sample with those of several separate normal controls that underwent independent hybridization (Levy & Wapner, 2018).
CMA offers high diagnostic performance due to the high resolution of the genome sequence that can be screened every ~750 kb, a definition that neither a conventional nor high-resolution karyotype can reach (Ahn et al., 2015; Edelson et al., 2019). On the other side of high resolution, the next generation sequencing (NGS) strategies, including the WES and WGS, respectively, have significant limitations for CNVs characterization as their application depends on bioinformatics algorithms, substantially limited by coverage and type of the sequence nucleotide content, among other aspects (Shaikh, 2017).
CNVs are a common cause of genetic diseases. In 1 of 200 live births, deletion or duplication causes neurodevelopmental delay (Smajlagić et al., 2021). CMA workup performance for the clinical diagnostic of neurodevelopmental delay and congenital anomalies is between 5% and 15% (Edelson et al., 2019), highlighting the importance of continuous application and evaluation of the diagnostic performance of the CMA This study aims to define the prevalence of CNVs by applying CMA in a Colombian clinical cohort of 3252 patients recruited over 5 years (2016–2021). To our knowledge, this is the largest clinical cohort reported for a Latino population.
2 MATERIALS AND METHODS
2.1 Type of study and patient selection
A retrospective observational cohort study of clinical cases was implemented through a full review of CMA tests performed on patients with a suspected genetic disease without a previously defined etiological diagnosis, regardless of age and sex.
The most frequent clinical conditions studied were a neurodevelopmental delay, intellectual disability, and autism spectrum disorder (ASD). These patients were evaluated by different multidisciplinary medical groups who, using international scales, diagnosed them with each of these conditions. The pediatric neurology group identified patients with neurodevelopmental delay using scales such as the Bayley Scales of Infant and Toddler Development and the denver developmental screening test (Jeong et al., 2017). For patients with intellectual disabilities, the assessment was performed by the neuropsychology group, which used the WISC-IV and the IQ test. Finally, ASD was diagnosed using scales such as the Social Responsiveness Scale, the Autism Spectrum Rating Scale, and the Early Screening for Autism and Communication Disorders (Li et al., 2018; Wetherby et al., 2021). It should be noted that all patients had been previously diagnosed with these conditions by the time CMA was requested. The ascertainment place was the “Specialized Laboratory of the Colombia Universitaire Clinic” (Bogotá, Colombia), where samples were collected and processed for 5 years (May 2016–November 2021). The entire CMA test sample allowed us to ascertain 3380 results (one per patient). Additionally, clinical records from patients were collected through workups completed during sampling. This clinical information is provided by the patient or guardian or by online documentation in the institutional electronic medical record system. Patients with an incomplete clinical history or without the requested CMA study results were excluded. The diagnostic yield was assessed in terms of definitive genetic diagnoses, inconclusive diagnoses, and patients with a CMA reported as normal.
2.2 The chromosomal array analysis
A peripheral blood sample was taken from each patient in a tube with EDTA anticoagulant for the chromosomal array study. DNA was extracted by a standard salting-out method, and samples were processed with the Affymetrix CytoScan 750 K oligo-SNP platform (afterward owned by Thermo Fisher Scientific) using a microarray containing 750,436 probes, including 550,000 non-polymorphic probes and 200,436 SNP markers, and analyzed with the Chromosome Analysis Suite (ChAS) Software.
2.3 Interpretation of CNV
All the CNVs were interpreted according to the currently updated criteria defined by the American College of Medical Genetics (ACMG), and CNVs were classified as a pathogenic, likely pathogenic, variant of uncertain significance (VUS), likely benign, and benign. Only when reporting the first three types of variants the type of found CNV, either deletion or duplication, was discriminated in the report, as well as the size (Mb), map location, OMIM gene content (number and type of genes involved), phenotypic associations, the inheritance pattern (de novo—whenever it was possible to evaluate the parents—or familial), as well as a comprehensive correlation with the available clinical information. The classification was performed using the available databases: DECIPHER, DGV (Database of Genomic Variants), ClinVar-National Center for Biotechnology, the technical standards for the interpretation and reporting of constitutional copy-number variants recommended by the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen; Riggs et al., 2020) and the current scientific knowledge at the time of analysis.
2.4 Conclusive genetic diagnosis
A genetic diagnosis was defined after identifying deletions or gains classified as pathogenic or likely pathogenic, correlating with patient phenotype or clinical suspicion.
2.5 Inconclusive outcomes
The inconclusive outcomes were defined as identifying a variant with unknown clinical significance, that is, the clinical geneticist cannot conclude a diagnosis because of the kind of variant or the insufficient scientific and clinical information.
2.6 Normal results
In this section, all patients with results without CNV were included, although this does not rule out a possible genetic etiology.
2.7 Statistical analysis
This observational study reported categorical variables as absolute frequencies or percentages, and continuous variables were presented through central tendency and dispersion measures.
The study was conducted following the Declaration of Helsinki and approved by the Institutional Ethics Committee of “Fundación Universitaria Sanitas (protocol code 565 of July 24th of, 2015) for studies involving humans.
3 RESULTS
A total of 3380 patients with available CMA were included in the study, of which 1327 were female and 2053 male (39.30% and 60.70%, respectively). The age of the cohort ranged from 0 to 69 y/o, with a high frequency of pediatric patients, n = 2500 (aged 0–14 y/o, median age = 6 y/o; Table 1). Evaluated patients arrive from disparate geographical regions of the Colombian territory, that is, 67.95% from Bogotá; 8.85% from Bucaramanga; 6.05% from the Caribbean coast cities (Cartagena, Barranquilla, and Santa Marta); and 4.29% from Villavicencio. Other cities with recurrent referrals were Ibagué, Valledupar, and Medellín. A total of 663 CMA tests reported at least one CNV (regardless of the classification type), with a CNV detection rate of 21.27% per patient (Table 1).
| Overall | Pediatric patients | Juvenile/adult patients | |
|---|---|---|---|
| Number of patients | 3380 | 2715 | 665 |
| Male | 2053 | 969 | 307 |
| Female | 1327 | 1746 | 358 |
| Mean Age | 10 | 6 | 28 |
| Median Age | 6 | 5 | 26 |
| Report type | |||
| Normal | 2661 | 2120 | 541 |
| Abnormal | 719 | 595 | 124 |
| CNV detection per patient | |||
| CNV Detection rate (%) | 21.27 | 21.92 | 18.65 |
| Diagnostic yield (%) | 11.56 | ||
The clinical spectra of phenotypes that generated CMA study requests included: neurodevelopmental delay (36.02%), autism (11.40%), intellectual disability (4.17%), and congenital abnormalities (4.03%; Table 2). Of the 663 patients with abnormal results, 65 (9.80%) had more than one CNV, and 61 carried at least one CNV classified as pathogenic or likely pathogenic, while in other cases (n = 4), all the reported CNVs were classified as VUS.
| Phenotype | n | % | Phenotype | n | % |
|---|---|---|---|---|---|
| Neurodevelopmental delay | 259 | 36.02 | Abnormality of brain morphology | 7 | 0.97 |
| Autism | 82 | 11.40 | RASopathies | 5 | 0.70 |
| Epilepsy | 40 | 5.56 | ADHD | 4 | 0.56 |
| Facial dysmorphism | 32 | 4.45 | Obesity | 4 | 0.56 |
| Intellectual disability | 30 | 4.17 | Intrauterine growth retardation | 4 | 0.56 |
| Short stature | 29 | 4.03 | Immune disorders | 4 | 0.56 |
| Congenital abnormalities | 29 | 4.03 | Musculoskeletal disorders | 4 | 0.56 |
| Heart diseases | 23 | 3.20 | Premature birth | 3 | 0.42 |
| Hypotonia | 20 | 2.64 | Ambiguous genitalia | 2 | 0.28 |
| Microcephaly | 12 | 1.67 | Hearing impairment | 2 | 0.28 |
| Kidney disorders | 10 | 1.39 | Infertility | 2 | 0.28 |
| Cerebral palsy | 7 | 0.97 | Other clinical indications | 49 | 6.82 |
| Total | 663 patients | ||||
We detected 773 CNVs, 58.73% corresponding to genomic gains, of which 23.15% were classified as pathogenic or likely pathogenic and 35.58% as VUS. The other 41.27% of CNVs correspond to losses, of which 31.43% were considered abnormal, and 9.84% were classified as VUS. Abnormal gains in autosomes represent 19.53% and losses 29.10%, while VUS gains and losses correspond to 31.30% and 8.79%, respectively. In the sex chromosomes, 3.62% of the CNVs correspond to abnormal gains (4.26% VUS), while abnormal losses represent 2.32% (1.03% VUS; Table 3). Additionally, 57 chromosomal arrays had some type of finding for patients with diagnoses of CNV-associated disorders requiring confirmation (34) and for the parents or children of patients with these characteristics (23). However, these were not considered for the calculation of diagnostic yield (Table 3).
| Overall | Pathogenic | Likely pathogenic | VUS | |
|---|---|---|---|---|
| Total CNVs detected | 773 | 349 | 73 | 351 |
| Gains | 454 | 137 | 42 | 275 |
| Autosomes | 393 | 117 | 34 | 242 |
| Sex chromosomes | 61 | 20 | 8 | 33 |
| Losses | 319 | 212 | 31 | 76 |
| Autosomes | 293 | 194 | 31 | 68 |
| Sex chromosomes | 26 | 18 | 0 | 8 |
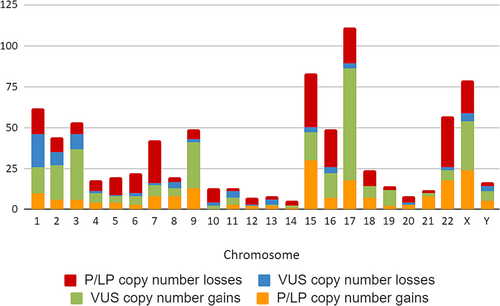
Figure 1 shows the number of CNVs found in each chromosome and their classification. Chromosomes 1 (7.47%), 15 (10%), 17 (13.37%), 22 (6.87%), and X (9.52%) show the highest number of copy number variants, although there is no clear differentiation of a generalized higher prevalence of any of the two types of variants.
The most frequent abnormal findings are summarized in Figure 2, which lists the number of abnormal gains and losses per locus. Among these, 17 losses (2.04%) and two gains (0.24%) were identified at the 7q11.23 locus. All were classified as pathogenic or likely pathogenic; the most frequent clinical correlation assigned to patients carrying this CNV was Williams syndrome. On the other hand, at the 15q11.2 locus, 13 abnormal gains (1.56%) and 15 abnormal losses (1.80%) were found out of a total of 20 and 16 CNVs, respectively; intellectual disability was the primary clinical correlation in these patients carrying gains harbored in this locus, while short stature and neurodevelopmental delay were clinically associated to the losses found; autism was also a frequent reason for study in patients with both findings. In chromosome 15, abnormal gains were also found in the 15q11.2q13.1 locus, when the study's main objective was to rule out Angelman and Prader-Willi syndromes. In addition, 3 (0.36%) of the 10 abnormal losses in this locus were found in patients with hypotonia.
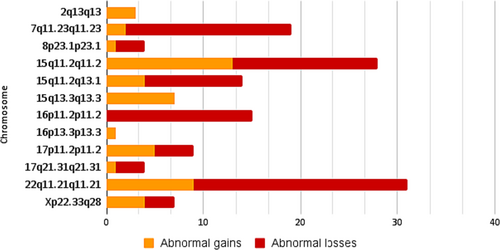
Another frequent CNV in patients with neurodevelopmental delay was abnormal losses at the 16p11.2 locus, accounting for 1.92% of the findings. In chromosome 22q11.21, losses and gains were mainly considered abnormal, diagnoses were associated with DiGeorge syndrome and Chromosome 22q11.2 microduplication syndrome, and the clinical correlation of patients with these findings included neurodevelopmental delay, epilepsy, and facial dysmorphism. Finally, four abnormal gains and three losses involving the entire chromosome were found in the X chromosome, accounting for 0.48% and 0.36% of the CNVs.
Other frequently found CNVs included those listed in Table 4; a high frequency (4.85%) characterizes these in the population studied (164/3380). Among these were CNVs at 2q13, of which 14 were gains and 8 were losses, representing 3.05%; the initial classification of these variants was VUS. At loci 3p25.2 (3.19%), 9q34.3 (3.05%), and 16p13.3 (1.94%), mainly gains were found, which out of 66, 59 (8.20%) were classified as VUS. Finally, gains at the 17q21.31 locus constituted 10.29% of the findings, 9.73% were classified as uncertain significance variants, and only 0.48% were classified as pathogenic or likely pathogenic.
| Locus | Overall | Abnormal gains | VUS gains | Abnormal losses | VUS losses |
|---|---|---|---|---|---|
| 2q13 | 25 | 3 | 14 | 0 | 8 |
| 3p25.2 | 25 | 2 | 23 | 0 | 0 |
| 9q34.3 | 25 | 1 | 22 | 1 | 1 |
| 16p13.3 | 15 | 1 | 14 | 0 | 0 |
| 17q21.31 | 74 | 1 | 68 | 3 | 2 |
CNVs gains, harbored in the 2q13 locus (2 out of 17 gains), with a size of 0.48 Mb that encompassed the RGPD6, MALL, and NPHP1 genes, were first classified as likely pathogenic. However, given the current scientific evidence, and the presence of this variant in databases such as DGV, it is now possible to reclassify it again as a probably benign variant.
4 DISCUSSION
CNVs were identified in 21.27% of the clinical cases evaluated, and 11.56% were established as the most likely cause of the patient's condition. The rate of abnormal findings among the patients with at least one CNV for the most frequent clinical indication (neurodevelopmental delay) was 64.61% (168/259), for intellectual disability 66.66% (20/30), for congenital malformations 34.48% (10/29), and for other frequent disorders such as epilepsy and heart diseases: 50% (20/40) and 47.82% (11/23), respectively. These figures support the importance of the application and evaluation of CMA as one of the most relevant genetic tools to dissect the molecular basis of a suspected genetic disorder, especially in patients with neurodevelopmental delay, intellectual disability, autism, and multiple congenital anomalies as suggested by Pinheiro et al. (2020)) and Chong et al. (2014)).
The detection rate and diagnostic yield of CMA in this evaluated cohort of 3380 patients are consistent with that reported in the worldwide literature, that is, the study by Pinheiro et al. (2020)) in which a cohort of 215 patients with global neurodevelopmental delay and intellectual disability was evaluated, reported a diagnostic yield of 23.3%; or the study carried out by Chong et al. (2014)) who reported abnormal findings in 19% of the 105 patients evaluated with intellectual disabilities, developmental delay, autism, and multiple congenital anomalies. Our cohort is at least 10 times bigger than these two previously reported cohorts indicating that these results are reliable. It is essential to clarify that we evaluated the overall diagnostic yield of the chromosomal microarray (CMA) and did not determine the diagnostic yield by pathology or by phenotypic condition. Even though we had primarily patients affected with neurodevelopmental disorders, some, either phenotypes or conditions, had a sporadic casuistic generating noise to conclude on such limited statistic.
The CNVs most frequently found were gains, which accounted for 58.31% of the findings. However, as previously described in the literature (Truty et al., 2019), CNV losses account for the highest proportion of pathogenic or likely pathogenic results (32.16%). In comparison, abnormal gain numbers only stood at 23.13%. Haploinsufficiency is one of the reasons why deletions tend to have a higher risk of pathogenicity since, in some cases, one copy of the gene is insufficient to generate a fully functional product and leads to abnormal phenotypes (Huang et al., 2010). In addition, factors such as the size of the deletions affect its pathogenicity since the number of genes involved depends on it.
One of the most remarkable findings of this study represents the persistent characterization of CNVs classified as VUS since this information provides the clinician with an important tool to define clinical diagnosis and allow familial genetic counseling. As a taste, for example, CNVs in the 2q13 locus that encompassed the RGPD6, MALL, and NPHP1 genes, were classified as likely pathogenic (which is not significantly different from 10 other similar findings reported as VUS), allowing us to reclassify them as VUS. Given that ClinGen gives the 2q13 recurrent region (includes NPHP1) [GRCh37] Chr2:110,862,108–110,983,703 a triplosensitivity score of 40, it is unlikely the existence of dosage sensitivity and therefore a better classification may be benign/likely benign (depending on the actual location, size, and gene content). On the other side, deletions of this region, the NPHP1 gene are associated with autosomal recessive Joubert syndrome, juvenile nephronophthisis, and Senior-Loken syndrome. Full gene deletions of NPHP1 have been well characterized, and ClinGen gives the 2q13 recurrent region (includes NPHP1) [GRCh37] Chr2:110862108–110,983,703 a haploinsufficiency score of 30, meaning it is more likely to be associated with the pattern of an autosomal recessive disease. Therefore, it could be better to classify these patients with deletions of this region as carriers of a pathogenic deletion (again, depending on the actual location, size, and gene content).
Similarly, deletions in this region encompassing LIMS1, RANBP2, CCDC138, and EDAR genes were recently associated with a predisposition to developmental delay and behavioral problems. Further, gains and losses of uncertain significance in this locus involve, in addition to MALL and NPHP1, the RGPD6 gene, frequently found in patients with epilepsy or language disorders. These results grant further analyses to evaluate this genomic region in a more significant bigger cohort.
At the 3p25.2 locus, all the gains found encompassed the RAF1 gene, and two included CAND2. In the literature, microduplications involving RAF1 have been associated with Noonan syndrome, characterized by congenital heart defects, short stature, and craniofacial dysmorphisms (n). However, out of 25 patients with this finding, only 7 showed facial dysmorphism with neurodevelopmental delay, and 2 had short stature without other additional malformations, suggesting a highly variable phenotype expressivity. In this case, the size of the duplication is not considered a determining factor in the pathogenicity of CNV since the median length of the reported cases is 0.185 Mb, which does not differ from previously reported cases in patients with all the characteristics of the phenotype and whose duplication involved the same genes (Luo et al., 2012).
Meanwhile, at the 16p13.3p13.3 locus, 15 CNVs were found, of which 14 were VUS gains. Unlike other frequently found CNVs, the duplications at this locus were highly heterogeneous; the sizes ranged between 0.025 and 0.9 Mb, encompassing the LMF1, SOX8, TSC2, and CACNA1H genes without a specific pattern. Interestingly, the clinical indication for CMA in 6 of these patients with this duplication was autism, so it would be worthwhile to study further the relationship of the gains at 16p13.3p13.3 with this condition in Colombian cohorts.
As previously mentioned, CNVs at the 17q21.31 locus constitute a significant proportion of the findings (10.29%). All the found VUS gains encompassed the KANSL1 gene, and their sizes ranged from 0.091 to 0.105 Mb, while those considered abnormal also included the SPPL2C, STH, MAPT, and CRHR1 genes and were more extensive than 0.5 Mb in size, this, in conjunction with the currently available evidence, allows us to reclassify the variant as probably benign. Besides, CNV gains involving KANSL1 are widespread in the general population and mostly classified as benign/likely benign in agreement with the actual knowledge. Clinical indications of patients with specific CNVs in 17q21.31 include a broad spectrum of disorders such as autism, neurodevelopmental delay, heart disease, cognitive disability, facial dysmorphism, epilepsy, congenital malformations, and short stature. Given the high variability of patient phenotypes, the lack of evidence supporting the CNVs' pathogenicity, and the frequency of CNV in the population harbored at 17q21.31. Further molecular studies are needed to define the real significance of these CNVs.
Similarly, patients with gains found at locus 9q34.3 had high heterogeneity in their phenotypes, including congenital malformations, epilepsy, hypotonia, neurodevelopmental delay, ambiguous genitalia, and prematurity. Most involved the same genes (FBXW5, C8G, LCN12, PTGDS, CLIC3, ABCA2, FUT7, NPDC1, ENTPD2, SAPCD2, MAN1B1, DPP7, and GRIN1). Therefore, new samples and analysis will allow us to define the phenotype underlying this locus and the population variability of CNVs harbored in this genomic region. Information provided in the Supplementary Material (Table S1) regarding genomic coordinates could be insightful to define minimal critical regions associated with pathogenic phenotypes and genotype variability at the populational level.
In summary, from 3380 patients tested with CMA, with a remarkable clinical heterogeneity, the diagnostic yield was 11.56%. By discriminating the cohort into more specific phenotypes, that is, including phenotypes such as neurodevelopmental delay (intellectual disability and autism), the rate of abnormal findings among patients with CNVs was 60.75%. In the case of epilepsies, it was 50%.
These figures remark on the importance of CMA as an essential tool to define the molecular basis of genetic diseases. We identified two frequent VUS CNVs that do not explain the patient's clinical phenotype because CNVs were homogeneous while the phenotype is highly variable. In this vein, gains in 17q21.31 with a range between 0.091 and 0.105 Mb affecting the KANSL1 gene and 9q34.3between 0.080 and 0.4 Mb cannot be considered the final etiological cause of the patient's phenotypes, and eventually, there will be considered either as likely benign o benign CNVs, even though for these patients is recommended to apply other molecular diagnostic strategies such as whole-exome sequencing and trinucleotide repeat expansion to reclassified these to CNVs gains.
It is crucial for the long-time patient follow-up, that is, years, to identify additional clinical features shaping the phenotype outlined by these CNVs as defined by CMA. The reclassification and new available scientific evidence from worldwide databases can help us to clarify whether the etiology of the patient's clinical phenotype is attributable to these CNVs. Furthermore, to improve the diagnosis, additional family (segregation, linkage) and population-based studies will, in the future, allow us to refine minimal critical regions and outline the gene or genes harbored in these regions involved by these CNVs and potentially underpinning abnormal phenotypes to improve the genetic counseling, and, eventually, the treatment and follow-up of these patients and their family.
AUTHOR CONTRIBUTIONS
Conceptualization: Juan Javier López-Rivera, Luna Rodríguez-Salazar, Paula Rueda-Gaitán. and Mauricio Arcos-Burgos. Methodology: Yina D. Carrillo and Orlando Gualdron. Software: Yina D. Carrillo. Validation: Yina D. Carrillo and Juan Javier López-Rivera. Formal analysis: Juan Javier López-Rivera, Luna Rodríguez-Salazar, Yina D. Carrillo, Paula Rueda-Gaitán. and Mauricio Arcos-Burgos; investigation, Juan Javier López-Rivera, Luna Rodríguez-Salazar, Yina D. Carrillo, Paula Rueda-Gaitán, and Mauricio Arcos-Burgos. Data curation, Luna Rodríguez-Salazar, and Paula Rueda-Gaitan; Writing—original draft preparation: Luna Rodríguez-Salazar, and Paula Rueda-Gaitan. Writing—review and editing: Mauricio Arcos-Burgos, Luna Rodríguez-Salazar, and Paula Rueda-Gaitan. Supervision: Juan Javier López-Rivera. Project administration: Juan Javier López-Rivera. Funding acquisition: Juan Javier López-Rivera. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
To the patients and families involved in this study.
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




