Characterization of seizures and EEG findings in creatine transporter deficiency due to SLC6A8 mutation
Myriam Abdennadher, Omar Khan, Luca Bartolini, Simona Bianconi present addresses are different from where the work was conducted.
Abstract
Seizures occur in up to 59% of boys with creatine transporter deficiency (CTD). While seizure phenotypes have been previously described, electroencephalogram (EEG) findings have only been reported in several case reports. In this prospective observational study, we report seizure characteristics and EEG findings in combination with neurobehavioral and SLC6A8 pathogenic variants in twenty males with CTD. Eighteen study participants (SP) underwent video-EEG, and seven had follow-up EEG recordings. Seizures typically occurred by age of 2 years. Thirteen (65%) had non-febrile seizures, requiring anti-seizure medications in nine. Four had febrile seizures. Seizures were bilateral tonic–clonic in 7 SP and focal impaired awareness in 5 SP; often responding to 1 to 2 antiseizure medications. EEG showed slowing in 5 SP, beta activity in 6 SP, and focal/multifocal, and/or generalized epileptiform activity in 9 SP. Follow-up EEGs in 7 SP showed emergence of epileptiform activity in 1 SP, and increased activity in 2 SP. In conclusion, seizures were frequent in our cohort but tended to respond to antiseizure medications. Longitudinal follow up provided further insight into emergence of seizures and EEG abnormalities soliciting future studies with long term follow up. Biomarkers of epileptogenicity in CTD are needed to predict seizures in this population.
1 INTRODUCTION
Creatine transporter deficiency (CTD) is an X-linked disease caused by pathological variants in SLC6A8 (Salomons et al., 2001), which codes for the sodium- and chloride-dependent creatine transporter 1 (CT1). Although CT1 is expressed ubiquitously, disrupting creatine transport to the muscle, kidneys, intestine, and brain, CTD manifests with a predominantly neurologic phenotype (Striessnig et al., 2020). Children have developmental delay leading to intellectual disability, behavioral problems such as ADHD or autism and failure to thrive (Miller et al., 2019). The disease may be a common cause of X-linked familial intellectual disability and is probably under-ascertained. Due to X chromosome inactivation females can be asymptomatic carriers or manifest with a wide range of severity (Van De Kamp et al., 2011). Urine screening may often reveal a high urine creatine to creatinine ratio in males, but not usually in females. However, in both males and females a decreased cerebral creatine peak can be observed using proton magnetic resonance spectroscopy (H-MRS). SLC6A8 sequencing has identified over 80 different pathogenic mutations, most often missense variants, causing creatine transporter deficiency disease (LOVD v. 3.0, n.d.; Betsalel et al., 2012). Creatine uptake studies in fibroblasts may also demonstrate abnormalities when gene sequencing or neuroimaging findings are unrevealing or inconclusive (Betsalel et al., 2012).
In addition to neurodevelopmental problems, seizures are common, occurring in 50%–59% of males with CTD (Miller et al., 2019; van de Kamp et al., 2013), although they are typically not the presenting symptom (van de Kamp et al., 2013). The current literature on EEG features is limited. EEG background may show slowing suggestive of non-specific cerebral dysfunction. Interictal epileptiform activity is usually focal, affecting more than one lobe (Battini et al., 2007, 2011). Generalized spike wave activity is less common and was associated with clinical eye and upper limb movement in one case report (Schiaffino et al., 2005). In this study, we expand the understanding of both the seizure natural history and EEG findings, often including overnight video-EEG monitoring, in 20 patients with well characterized CTD.
2 METHODS
Male study participants (SP) were enrolled in an observational study (17-CH-0020—Observational Study of Males with Creatine Transporter Deficiency (CTD), NCT02769949) and evaluated at the National Institutes of Health Clinical Center (NIH-CC), Bethesda, Maryland. The Institutional Review Board of NICHD approved the protocol. Ongoing ethical review was provided by the NIH Clinical Center IRB. Written informed consent was obtained from parents or legal guardians. All subjects underwent sequencing of SLC6A8. For this study, we included 20 participants who have genetically confirmed pathogenic variants and completed an EEG at the National Institutes of Health between January 2017 and October 2022.
Study subjects were assessed by pediatric geneticists for history, medications, general physical, and neurological exam. Additional evaluations included CBC and blood chemistry, lumbar puncture for glucose, protein, albumin, L-lactate, amino acids, structural MRI, H-MRS, and phosphorous MRS. Urine creatine to creatinine ratios were measured. Follow up visits were scheduled at approximately 6-month intervals.
2.1 Neurodevelopmental testing
All study subjects received a neurodevelopmental evaluation by psychologists during study visits with the Vineland Adaptive Behavior Scales, Third edition (Vineland-3) (Sparrow et al., 2016). The Vineland provides information about four domains of functioning: communication, daily living skills, socialization, and motor skills. Composite standard scores extracted from these domains are combined to obtain the overall adaptive score that has a mean of 100, standard deviation of 15, with scores below 70 used as one indicator of intellectual disability.
2.2 Electroencephalogram
Electrodes were placed by electroencephalogram (EEG) technicians with 18 channels following the international 10–20 system in compliance with Guideline One of the American Society of Clinical Neurophysiology. Video EEGs were recorded on Nihon Kohden machines. The EEGs were reviewed by at least two experienced electroencephalographers using a minimum of three different montages (bipolar, transverse, and referential).
2.3 Brain MRI
Brain MRI and MRS were performed using 3 Tesla Siemens MRI. Imaging was obtained under sedation.
3 RESULTS
Table 1 shows a summary of demographic, clinical, genetic, and EEG findings in this cohort. Twenty SP were included, with ages ranging from 3 to 17 years (median = 7 years and 4 months) at the time of first EEG. Median Vineland-3 score was 43.5 with a range of 22–65.
| SP | Age at most recent EEG | Vineland-3 ABC | SLC6A8 pathogenic variants | Seizure history | ASM | EEG | EEG duration (min) | f/u EEG findings | Brain MRI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Background | Epileptiform activity | |||||||||
| 1 | 11y7m | 31 | c.1667G>A; p.Trp556X | Febrile seizure | None | Limited test; no abnormalities noted | None | 25 | None | Left plagiocephaly |
| 2 | 6y11m | 49 | c.321_323delCTT; p.Phe107del | BH (birth-2y, resuscitation ×2) Staring spells (ongoing) Myoclonic seizure during PS, also rare IEDs at 3y |
None | Normal | None | 840 | None | Normal |
| 3 | 10y2m | 63 | p.Leu286Trpfs22 | Staring spells (1–8y) | None (h/o CBZ) | Normal | None | 936 | None | Nonspecific prominent liquid in optic sheets |
| 4 | 6y3m | 61 | c912+36_913-18del35 | FS | None | Intermittent R > L temporal beta activity | None | 932 | None | Mild thinning of the posterior part of corpus callosum |
| 5 | 3y11m | 59 | c912+36_913-18del35 | FS (6 m), SE (15 m) | None | Intermittent R > L temporal beta activity | None | 926 | None | Normal |
| 6 | 13y5m | 25 | p.Trp518X | BH->bilateral convulsive motor activity (9–12 m), BTCS (1–3y) | None (h/o LEV) | Not tolerated | – | 0 | None | Normal |
| 7 | 6y7m | 31 | p.Val358_Phe365del | Motor/BTCS (~3y-ongoing) SE (multiple, last at 6y) |
OXC, VPA | Diffuse excessive beta activity L frontocentral intermittent slowing |
Independent L, R or bifrontal spike and sharp waves | 1066 | None | Not done |
| 8 | 4y6m | 45 | p.Phe365Leufs*105 | None | None | Diffuse excessive beta activity maximal temporally | None | 964 | None | Left plagiocephaly Low myelination |
| 9 | 17y11m | 25 | c.1146_1150dup; p.Leu384ArgfsX14 | BTCS (onset 14y) | OXC | Normal | R > L frontal spike and waves | 952 | Bifrontal spikes; possible frontal electrographic seizures | Normala Delayed myelinationb |
| 10 | 4y6m | 49 | p.Gly466Arg | None | None | Not tolerated | – | 4 | None | Nonspecific bilateral hyperintensities |
| 11 | 4y2m | 61 | p.Phe408del | BH (4w, ongoing) “Sensory seizures” with brief stiffening | CBDc | Mild–moderate diffuse background slowing | L > R temporo-parietal sharp waves in sleep, dyshormia | 847 | None | Delayed myelination |
| 12 | 7y2m | 65 | 293 bp deletion of exon encompassing part of exon 10 and entire exon 11 | BTCS (6y) Staring spells (6y) |
OXC | Mild diffuse background slowing; diffuse excessive beta activity; L temporal slowing | R temporal and occipital spikes; dyshormia | 420 | 2nd, 3rd EEGs: increased multifocal IEDs | Nonspecific bilateral hyperintensities |
| 13 | 10y5m | 58 | p.Tyr475-Ala478delinsTer | None | None | Mild diffuse background slowing | Broad L predominant spikes in sleep (dyshormia) | 950 | None | Normal |
| 14 | 9y5m | 28 | c.1135 C>T; p.Pro382Leu | None (limited history before 4 years) | None | Bitemporal excessive beta activity; frontal intermittent rhythmic delta activity | Bifrontal sharp waves; dyshormia | 995 | Similar; bifrontal spikes and sharp waves | Corpus callosum thinning Nonspecific bilateral frontotemporal hyperintensities |
| 15 | 5y9m | 37 | p.Phe408del | BTCS ×2 (19 m) | LEV | Normal | None | 25 | Technically limited, normal 4 h EEG | Not done |
| 16 | 9y9m | 57 | P.Gly322Trp | None | None | Normal | None | 960 | Normal overnight EEG | Normal |
| 17 | 12y4m | 39 | c.1254+1G>A; IV58+1G>A | FS (2y) BH (until 3y) “Stiffening” episode (8y) |
None | Normal | Generalized spike and waves | 960 | Similar: bifrontal IEDs on overnight, normal routine EEG | Not done |
| 18 | 7y1m | 35 | c.321_323delCTT; p.Phe107del | Myoclonic jerks Staring spells (onset 1y) |
LEV GBP |
Mild diffuse background slowing, bilateral parieto-occipital focal slowing | L temporo-parietal spike and polyspike and waves | 900 | Similar; bilateral parieto-occipital spikes | Not done |
| 19 | 14y1m | 22 | c.321_323delCTT; p.Phe107del | SE (8y) Staring spells (ongoing) |
LEV OXC GBP |
Mild diffuse background slowing | None | 60 | None | Not done |
| 20 | 7y6m | 43 | c.1222_1224delTTC p.Phe408del |
BTCS (20 m) | None (h/o LEV) | Normal | L parietal spike and waves; Generalized spike and waves with PS |
25 | None | Normal |
- Abbreviations: BH, breath-holding; BTCS, bilateral tonic–clonic seizure; CBD, cannabidiol; CBZ, carbamazepine; FS, febrile seizure; L, left; LEV, levetiracetam; m, month; n/a, not applicable; OXC, oxcarbazepine; PS, photic stimulation; R, right; SE, status epilepticus; SP, study participant; VPA, valproic acid; y, year.
- a On current MRI.
- b On an earlier outside MRI.
- c Not prescribed for seizure treatment.
We obtained a history of paroxysmal events in 75% of our cohort of 20 CTD patients. Four SP (20%) had a history of febrile seizures, including one (SP 5) who later developed afebrile status epilepticus (no epileptiform activity on study EEG) and another (SP 17) who had breath holding spells and may have had a non-febrile seizure later (generalized spike and wave on study EEG). Thirteen out of twenty CTD patients (65%) had a history of at least one non-febrile seizure including nine who were treated with an antiseizure medication for epilepsy. Onset of non-febrile seizures occurred at less than one year of age in three, six at one to two years old, four from 2 to 8 years old, and at 14 years in one SP (Figure 1) (median age 1.7 years). Seizure semiology was obtained by history, including “staring spells” in five, bilateral tonic–clonic seizures in six, a brief tonic seizures in two and possibly in another participant (SP 17), myoclonic seizures in two, including one with a myoclonic seizure induced during photic stimulation on EEG, and status epilepticus in three SP. We did not find a difference in neurodevelopmental performance (Vineland-3) of participant with a history of a non-febrile seizure compared to those without (mean with = 42.5, mean without = 47.2, p = 0.49).
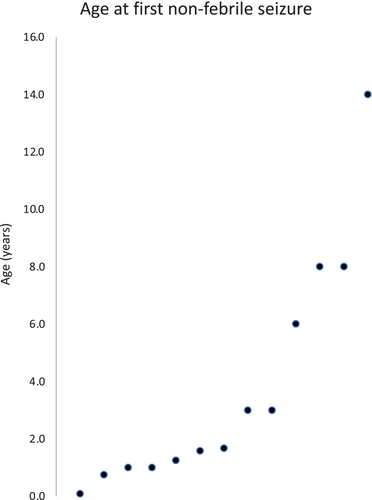
Breath-holding spells were reported in four SP from birth to 4 years of age, with two reportedly immediately followed by a possible seizure (tonic–clonic seizure in SP 6, and tonic in SP 17), one with a previous history of a perinatal tonic seizure (SP 11), and one who later developed epilepsy (SP 2). Of the nine SPs treated with antiseizure medications (ASMs), eight required one to two ASMs and one was treated with three ASMs. Three had seizure recurrence with ASM withdrawal. Levetiracetam and oxcarbazepine were the most commonly prescribed ASMs (in four SP each). One SP was on cannabidiol that was not prescribed for seizure treatment.
3.1 EEG findings
Fourteen participants were able to complete a baseline overnight video-EEG study (duration range 7–17.7 h). Four SP completed a routine EEG only (25–60 min). Two participants did not tolerate the procedure. Baseline EEG showed diffuse background slowing in four SP and excessive diffuse beta activity in six SP. Interictal epileptiform discharges (IEDs) were found at baseline or follow up EEG in nine SP, with focal IEDs (1–2 foci) in ten SP, multifocal IEDs (>2 foci) in two SP (focal at baseline becoming multifocal at 3 and 9 years follow up, SP 12 and 9, respectively), and generalized IEDs in two SP. One SP had generalized epileptiform activity provoked by photic stimulation (SP 20). Similarly, a photo-convulsive myoclonic seizure with generalized spike and wave was reported on another SP (SP 2) on an outside EEG recording. There was no association between IEDs patterns and seizure semiology; IEDs were focal in SP with FIAS (2), BTC (4), and myoclonic seizure (1), and were generalized in SP with FIAS (1), BTC (2), and tonic (1) seizure. Yield appeared to be higher in the longer overnight EEG recordings, with epileptiform activity identified in 1 of 4 (25%) routine EEG studies and 8 of 14 (57%) overnight (prolonged) EEGs, despite a history of seizures treated with ASMs in ¾ patients with routine EEGs. Of four patients with no history of paroxysmal events, IEDs were detected in two. Conversely, seizures were reported in seven of nine patients without IEDs on the baseline EEG recording, although only two of these SPs were being actively treated for ongoing seizures. Detailed EEG findings are reported in Table 1 and Table S1.
We were able to obtain follow-up EEG recordings in 7 out of 18 SP with baseline EEG recordings. Compared to baseline, follow-up EEG showed evolution of focal to generalized epileptiform activity in two (SP 9, 12), while interictal activity remained similar in three SP (SP 14, 17, 18). Two SP had normal baseline and follow up EEGs (SP 15, 16). Of note, in two patients routine EEGs obtained in follow-up were normal following abnormal overnight EEGs. It cannot be determined whether this represents an improvement or decreased sensitivity of routine EEGs in detecting infrequent or state-related abnormalities. Detailed findings on baseline and follow-up EEG are summarized in Table S1.
3.2 Brain MRI
Abnormalities from clinical reads of brain MRI obtained in 15 males included delayed myelination in two patients (in two SP). Nonspecific findings were plagiocephaly (in two SP), non-specific bilateral T2 hyper intensities (in three SP), corpus callosum thinning (in two SP), and prominent liquid in optic sheets (in one SP).
4 DISCUSSION
This study presents seizure and EEG findings as well as genetic, developmental, and imaging features in 20 patients with CTD. Similar to previous cohorts, we found that seizures were common in CTD, encompassing febrile and afebrile seizures. Although seizures were typically infrequent and well controlled with anti-seizure medications, three had a history of at least one episode of status epilepticus. Here, we add to the literature by describing the EEG findings in these patients. Compared to routine EEGs, overnight EEGs appear to be more sensitive in detecting IEDs, which were typically focal but at times appeared more generalized. Follow-up EEG recordings typically yielded similar results, although on occasion demonstrated additional foci of IED activity.
In our overall cohort, we obtained a history of paroxysmal events in 75% of our participants. We obtained a history of febrile seizures in four SP (20%) and breath-holding spells in four SP (20%). At least one afebrile seizure was reported in 13 patients (65%). The most commonly reported seizure type was bilateral tonic–clonic (6/20), while staring spells were reported in 5/20. Four out of five participants with staring spells had additional seizure types, three had unremarkable EEGs, and two had focal IEDs, making it challenging to distinguish whether these episodes represent generalized absence seizures or focal seizures with impaired awareness. Three patients had a history of status epilepticus, two had brief tonic seizures, and two had a history of photic stimulation-induced myoclonic seizures. In patients with likely clinical seizures, onset typically occurred by the age of 4 years, and often within the first 2 years of life (median 1.7 years), similar to other studies (Battini et al., 2007; Miller et al., 2019; Valayannopoulos et al., 2012; van de Kamp et al., 2013). Similar to previous reports, seizures in our cohort tended to be relatively infrequent and well-controlled, responding to one antiseizure medication in most cases (Van De Kamp et al., 2012; van de Kamp et al., 2013).
The prevalence of non-febrile seizures is higher in our study population compared to previous work (40%–59% including febrile and non-febrile seizures) (Miller et al., 2019; Van De Kamp et al., 2011). Previous studies have reported that febrile seizures often preceded the onset of recurrent non-febrile seizures (Battini et al., 2007; Leuzzi et al., 2013; Margherita Mancardi et al., 2007; Valayannopoulos et al., 2012; van de Kamp et al., 2013) but this was not reflected in our cohort. This difference may be related to recruitment bias, as some families with children with possible seizures could have been more interested in participating in our study that includes EEG evaluation. Alternately, paroxysmal events in infancy can be difficult to assess and/or diagnose retrospectively when relying on parental reports and may also alter the reported seizure frequency. For instance, there were a number of patients with reported breath-holding spells in this cohort, most of whom also demonstrated EEG abnormalities, particularly in overnight recordings, suggesting a possible but not definite epileptic etiology for these events.
Epileptiform abnormalities were observed in 9 of 18 SP who underwent EEG recordings. Yield appeared to be higher in the longer overnight EEG recordings, with epileptiform activity identified in 1 of 4 (25%) routine EEG studies (25–60 min duration) and 8 of 14 (57%) overnight (prolonged) EEGs. Epileptiform activity in this cohort was predominantly focal, involving one to two foci in 10 SP. To our knowledge, EEG findings have been described previously only in isolated case reports (Battini et al., 2007; Leuzzi et al., 2013; Mancini et al., 2005; Margherita Mancardi et al., 2007; Schiaffino et al., 2005), of which two similarly reported focal epileptiform activity (Battini et al., 2007, 2011). Multifocal and generalized epileptiform activity (in two SP each) were less frequent in our cohort, although each has been previously described in case reports (Leuzzi et al., 2013; Mancini et al., 2005; Schiaffino et al., 2005).
We were able to obtain follow-up EEG recordings in 7 out of 18 participants. Longitudinal EEG findings showed emergence of epileptiform activity in one patient (SP9) who had a reportedly normal outside EEG, increased epileptiform activity in two, and no changes in five, of which two had similar epileptiform activity and three had normal EEGs. Similarly, Van De Kamp et al. (2012) described the emergence of epileptiform activity in the EEGs of two out of nine individuals followed during a treatment cohort. We acknowledge that these findings are limited by the variable follow up intervals and EEG duration. Future prospective longitudinal studies investigating neurophysiological changes prior to and following seizure onset in CTD patients are needed, and may lead to identifying early biomarkers of epileptogenicity to recognize patients who need early antiseizure medication treatment.
In our cohort, a history of breath-holding spells was reported in four participants, which has not previously been reported in CTD. Breath-holding occurred from birth to 4 years of age, requiring resuscitation in one patient, and additional afebrile seizures of differing semiologies were noted in each of these patients. EEG findings differed in each of these patients (not tolerated, normal, focal, and generalized IEDs), therefore not consistent necessarily with a common epileptic etiology. Breath-holding spells are not uncommon in the pediatric population and are thought to occur in up to 5% of otherwise healthy children, especially between the ages of 6 months and 6 years, and can be familial (DiMario, 2001). The exact mechanism of breath-holding spells has not yet been elucidated. The differential diagnosis of breath-holding spells may include both ictal events and possible cardiac causes, and a variety of factors have been implicated, including autonomic nervous system dysfunction, vagal hyperreactivity, delayed brainstem myelination, and iron deficiency with or without anemia (DiMario, 2001). In clinical practice, children with breath-holding spells do not typically undergo a workup for epilepsy or cardiac etiologies unless they present with atypical features. Rarely, severe breath-holding spells may result in cardiac conduction anomalies, including asystole (Legge et al., 2002). More research to investigate whether breath-holding spells in children with developmental delays or autism are more associated with cardiac or neurological issues than in general population is needed.
Creatine (⍺-N-methylguanidino acetic acid) plays a crucial role in providing energy after conversion into phosphor-creatine in high-energy demanding organs: brain and muscles (Béard & Braissant, 2010; Wallimann et al., 1992). Mechanisms contributing to epileptogenesis in CTD are unclear. Animal studies have found increased accumulation of GAA in CTD; perhaps leading to epileptogenicity (Almeida et al., 2006). Other scholars have proposed a neuromodulation role to creatine (Braissant & Henry, 2008; Sijens et al., 2005). Creatine/phosphocreatine/creatine kinase system plays a major role in maintenance of cell membrane potential and ions gradient (Wyss & Kaddurah-Daouk, 2000). In addition, animal studies have found that creatine kinase, the enzyme that catalyzes the ATP-creatine phosphate system, is predominantly seen in hippocampal granular and pyramidal cells, and astrocytes; its distribution reflects energy needs for the maintenance of extracellular space homeostasis (Hemmer et al., 1994). Creatine double knock-out mice showed hippocampal atrophy and impaired spatial learning (Béard & Braissant, 2010; Jost et al., 2002; Streijger et al., 2005). These studies indicate a relation between creatine and maintenance of hippocampi; speculating a potential role of CTD in seizure susceptibility.
In this cohort, brain MRI showed delayed myelination, thin corpus callosum, and non-specific parenchymal hyperintensities. These findings are consistent with previous reports demonstrating a variety of non-specific abnormalities (Anselm et al., 2006; Bruun et al., 2018; De Grauw et al., 2003; Lion-François et al., 2006; Salomons et al., 2003; Schiaffino et al., 2005). MR spectroscopy (MRS) has been proposed to be useful in suspected CTD by measuring creatinine peak (low in CTD), or by measuring creatine to creatinine ratio in urine (Almeida et al., 2004).
Overall, this study adds data to the literature about the types of seizures and EEG findings in CTD. Our findings are limited by the relatively small number of participants and by retrospective descriptions of the clinical semiology of seizures, often relying on parents' reports, as well as the timing of the EEG recording in relation to the timing of the reported seizures and/or treatment with seizure medications. Our findings will need to be supported by additional longitudinal study to characterize the relationship between clinical seizures, global functioning, and EEG abnormalities.
5 CONCLUSION
In the literature, seizures occur in up to 59% of patients with CTD, while epilepsy is diagnosed in about one-third of males with CTD. Seizures might be under reported, given that we found that 75% of our participants had at least one possible seizure. Seizures are often controlled with one medication. Based on our study findings, video EEG may be normal or show focal, multifocal or generalized epileptiform activity with possible progression with the disease. Further longitudinal EEG studies will help clarify the natural evolution of the disease in males with CTD and also in female carriers. Further studies to understand both the exact role of creatine in brain metabolism, and how both the deficiency of creatine in CTD and GAMT lead to the high prevalence of seizures are needed.
AUTHOR CONTRIBUTIONS
Audrey Thurm: data collection, and writing-editing and review. Forbes D. Porter: funding acquisition, methodology, and writing-review and editing. Judith S. Miller: funding acquisition, methodology, data curation, and writing-review and editing. Luca Bartolini: data collection, formal analysis, and writing-editing and review. Myriam Abdennadher: conceptualization, data collection, formal analysis, original draft, and writing-review and editing. Omar Khan: data collection, conceptualization, and writing-editing and review. Samar Rahhal: data curation, formal analysis, and writing-review and editing. Sara Inati: conceptualization, data curation, formal analysis, and writing-review and editing. Simona Bianconi: conceptualization, formal analysis, and writing-review and editing. William Theodore: methodology, and writing-review and editing.
ACKNOWLEDGMENTS
We are grateful to the study participants, their families, and the support organizations for the motivation and inspiration they have provided. We thank colleagues and staff who enabled the conduct of this study and the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
Dr. Abdennadher reports funding from the Grinspoon award and BU CTSI grant for research unrelated to this manuscript. Dr. Bianconi and Dr. Miller report funding support as consultant for Ultragenyx. Drs. Bartolini, Inati, Khan, Porter, Rahhal, Theodore, and Thurm have nothing to disclose.
CLINICAL TRIAL REGISTRATION
NCT02769949.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




