A recurrent KCNK4 variant in a dominant pedigree with hypertrichosis and gingival fibromatosis syndrome: Variable phenotypic expressivity and insights on patients' dental management
Abstract
Abnormal hyperpolarization of the KCNK4 gene, expressed in the nervous system, brain, and periodontal ligament fibroblasts, leads to impaired neurotransmitter sensitivity, cardiac arrhythmias, and endocrine dysfunction, as well as, progressive cell proliferation. De novo gain of function variants in the KCNK4 gene were reported to cause a recognizable syndrome characterized by facial dysmorphism, hypertrichosis, epilepsy, intellectual/developmental delay, and gingival overgrowth (FHEIG, OMIM# 618381). FHEIG is extremely rare with only three reported cases in the literature. Herein, we describe the first inherited KCNK4 variant (c.730G>C, p.Ala244Pro) in an Egyptian boy and his mother. Variable phenotypic expressivity was noted as the patient presented with the full-blown picture of the syndrome while the mother presented only with hypertrichosis and gingival overgrowth without any neurological manifestations. The c.730G>C (p.Ala244Pro) variant was described before in a single patient and when comparing the phenotype with our patient, a phenotype–genotype correlation seems likely. Atrial fibrillation and joint laxity are new associated findings noted in our patient extending the clinical phenotype of the syndrome. Dental management was offered to the affected boy and a dramatic improvement was noted as the patient regained his smile, restored the mastication function, and resumed his psychological stability.
1 INTRODUCTION
The K+ channel subfamily K member 4 (KCNK4), also known as TRAAK (TWIK-related arachidonic acid-stimulated K+), is a member of the two-pore-domain potassium channel (K2P) family, which controls the resting membrane potential (Brohawn et al., 2012). Abnormal activation or inhibition of potassium (K+) currents across the cellular plasma membrane can alter the neurotransmission leading to endocrine and renal dysfunction, cardiac arrhythmias, and in some instances disrupted developmental processes (Bauer et al., 2018). The human KCNK4 is widely expressed in the brain, placenta, testis, small intestine, prostate, kidney, and periodontal ligament fibroblasts (Lesage et al., 2000; Mariani et al., 2021; Urrego et al., 2014).
An autosomal dominant neurodevelopmental disorder with facial dysmorphism, hypertrichosis, epilepsy, intellectual delay, developmental delay, and gingival fibromatosis (OMIM# 618381) was described by Bauer et al. (2018) in three unrelated patients harboring gain of function variants in the KCNK4 gene. Hypertrichosis and severe gingival enlargement adversely affect patients' life leading to esthetic, functional, and psychological problematic concerns (Buch & Ranganath, 2021; Gawron et al., 2016).
Herein, we describe an Egyptian family (a patient and his mother) with a recurrent KCNK4 missense variant with variable expressivity in regard to the associated neurological findings. We also suggest that this variant is responsible for a relatively milder phenotype and the presence of a possible phenotype–genotype correlation.
2 MATERIALS AND METHODS
2.1 Patients
A 5-year-old boy (Patient 1) with developmental delay and a history of seizures was referred to the Limb Malformation and Skeletal Dysplasia Clinic, National Research Centre (NRC), Cairo for genetic diagnosis and management. He was the only child born to a nonconsanguineous couple. The mother was similarly affected and the father was blind with a family history of retinal affection (Figure 1a). Gestational history was unremarkable. The patient was full-term and delivered by Cesarean section due to a large estimated birth weight. At birth, his weight was 4.5 kg (+1.9 SDS) and there was no history of incubation. Regarding developmental history, he had head support at the age of 3 months, sat at 8 months, and walked at 1 year and 6 months. Epileptic fits were noted at the age of 2 years in the form of generalized tonic colonic attacks that recurred every 2–3 months and were poorly responsive to antiepileptic drugs. Clinical examination of the patient revealed generalized hypertrichosis, dysmorphic facies in the form of narrow forehead, bitemporal narrowing, thick bushy eyebrows, deep set eyes, low set ears, short deep philtrum, everted upper lip, and low hairline in addition to generalized lax joints (Figure 1b,c). His weight was 21 kg (80th centile; 0.83 SDS), height was 115 cm (90th; 1.34 SDS), and head circumference was 52 cm (75th; 0.7 SDS). Radiological bone age was about 3 years and 6 months using the Greulich and Pyle Atlas. Neurological examination revealed mild hypotonia and normal deep tendon reflexes. Vineland scale revealed an IQ score of 75 and brain imaging showed wide cerebrospinal fluid (CSF) space. Eye evaluation was normal. Electroencephalogram revealed generalized epileptogenic activity. Electrocardiogram revealed atrial fibrillation. Echocardiography and abdominal pelvic ultrasound were normal.
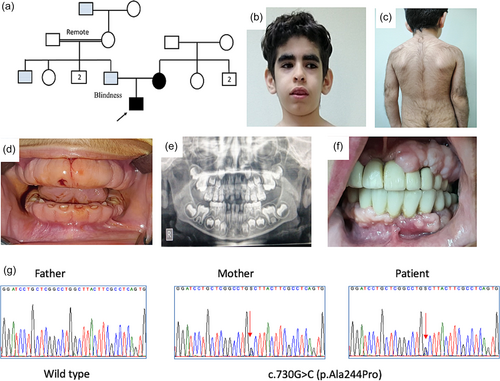
Oral examination of the boy revealed thick lips, thick gingiva covering the crowns of the teeth and preventing eruption of upper anterior teeth, the lower anterior deciduous teeth were embedded in the bulky overgrown gingiva. High-arched palate, bifid tip of the tongue, thick alveolar ridge, and microdontia were present as well. Panoramic radiographic view, at the age of 7 years, showed retained lower deciduous central incisors and delayed eruption of successors (Figure 1d,e).
The mother (Patient 2), 35 years old, had generalized hypertrichosis with a history of frequent facial and body hair removal, a hairy forehead, and long eyelashes in addition to gingival hypertrophy. Oral examination revealed macroglossia and mild gingival enlargement. The mother was previously subjected to a gingivectomy procedure at 13 years old with very mild recurrence of the gingival enlargement along years (Figure 1f). She was of average mentality and normal neurological examination with no history of seizures. Eye evaluation was normal. Electrocardiogram, echocardiography, and abdominal pelvic ultrasound were normal.
2.2 Dental management
Surgical dental management of the enlarged gingiva of the patient was suggested at the age of 7 years and 6 months and parents were informed about the surgical scenario and they signed an informed consent. For general anesthesia, the tube was placed nasally. The patient received SEVO flurione gas inhalation on flow rate of 2 L/min in a circle system. The patient was infiltrated at the site of the operative area with 1/200,000 adrenaline to control bleeding; with total dose of 50 μg on mml saline. The gingival tissue of both jaws was removed by dental electrosurgical cautery with intensity of 50 mA. The operative area was irrigated with saline and hydrogen peroxide 2%. After recovery, Amoxicillin/clavulanate antibiotic and nonsteroidal anti-inflammatory drugs were prescribed for 5 days to the patient. From the second day, the patient was instructed to gurgle with chlorhexidine mouth wash for a week. After 1 week of surgical operation and during follow-up, oral examination revealed anterior open bite, diastema, and microdontia. Soft acrylic guard was designated to the patient and worn overnight to avoid the recurrence of gingival enlargement. The soft acrylic guard was anteriorly cut to uncover the teeth in order not to interfere with teeth eruption (Figure 2). The patient was scheduled for follow-up every 2 months to assess the gingival condition.

2.3 Histopathological study
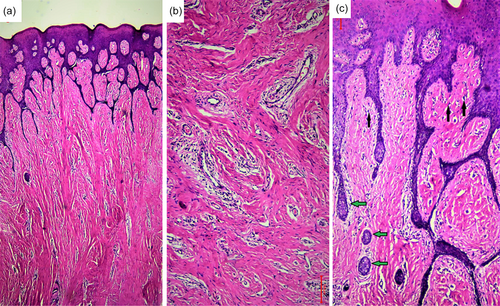
Histopathological examination of the excised gingival tissue revealed an increased accumulation of collagen fiber bundles by plump mature fibroblasts. There were some areas of hyalinization and numerous dilated blood vessels that were surrounded by perivascular edema. Moderate chronic inflammatory infiltrate was also present, especially around blood vessels. The covering surface epithelium was hyperplastic with long thin interlacing rete ridges. Moreover, pseudoepitheliomatous hyperplasia could be appreciated in some areas (Figure 3).
The detailed clinical, orodental, and molecular data of our patient and his mother in addition to the three previously reported patients are summarized in Table 1.
| Our patients | Bauer et al. (2018) | ||||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 (mother of Patient 1) | Patient 1 | Patient 2 | Patient 3 | |
| Age | 5 years | 35 years | 11 months | 5 years 7 months | 8 years |
| Sex | M | F | M | M | F |
| Parental consanguinity | − | − | − | − | − |
| FH of similar condition | + | + | − | − | − |
| Ethnicity | Egypt | Egypt | Italy | Italy | Europe |
| Motor development | Normal | Normal | Delayed | Normal | Delayed |
| Mentality | IQ score of 75 (borderline) | Normal | Delayed | IQ score of 85 (low average) speech delay | Delayed |
| Anthropometric data | Ht: 115 cm (90th) | NA | 78 cm (90th–97th) | 104 cm (50th) | 117.7 cm (10th) |
| Wt: 21 kg (80th) | NA | 9.0 kg (25th–50th) | 15.5 kg (10th) | 21.6 kg (10th) | |
| HC: 52 cm (75th) | NA | 43.5 cm (3rd–10th) | 51.2 cm (25th) | 51.5 cm (10th) | |
| Facies | Dysmorphic facies, bitemporal narrowing, hairy forehead, thick brushy eyebrows, synophrys, long eye lashes, everted Upper Lip, dimpled broad chin, large prominent low set ears, short neck, and low hair line | Hairy forehead, synophrus, long eye lashes, flat philtrum, and frequent removal of facial hair | Hypotonic facies, bitemporal narrowing, micrognathia, deep-set eyes, brushy eyebrows, long eyelashes, and low-set ears | Hypotonic facies, bitemporal narrowing, micrognathia, deep-set eyes, brushy eyebrows, long eyelashes, and low-set ears | Hypotonic facies, micrognathia, deep-set eyes, brushy eyebrows, long eyelashes, and low-set ears |
| Oro-dental findings | Short deep philtrum, thick incompetent lips, embedded teeth, high arched palate, thick alveolar ridge, anterior open bite and microdontia | Macroglossia, and highly attached labial frenum | Short deep philtrum, prominent upper and lower vermilion, and everted upper lip | Short deep philtrum, prominent upper and lower vermilion, and everted upper lip | Short deep philtrum, prominent upper and lower vermilion, everted upper lip, Pierre Robin sequence, and cleft palate |
| Eye evaluation | Normal | Normal | Nystagmus and bilateral optic hypoplasia | Normal | Nystagmus, and bilateral optic hypoplasia |
| Hypertrichosis | + | + | + | + | + |
| Gingival fibromatosis | + | + | + | + | + |
| Tone and reflexes | Mild hypotonia | Normal | Hypotonia and hyperreflexia | Normal | Hypotonia and intention tremor |
| Epilepsy/EEG | +/EEG showed generalized epileptogenic activity | −/Normal EEG | +/Disorganized pattern, some high voltage activity, and diffuse slow wave activity | +/Right focal clonic seizure with secondary generalization | Tonic clonic seizure |
| Brain image | Wide CSF space | ND | Thin corpus callosum and enlarged ventricles | Mild enlargement of bilateral frontal-insular and temporal subarachnoid spaces | − |
| Other | Atrial fibrillation and generalized joint laxity | − | − | − | Brachydactyly and congenital hip dysplasia |
| KCNK4 variant (NM_001317090.1) | c.730G>C (p.Ala244Pro) | c.730G>C (p.Ala244Pro) | c.515C>A (p.Ala172Glu) | c.730G>C (p.Ala244Pro) | c.515C>A (p.Ala172Glu) |
- Abbreviations: CSF, cerebrospinal fluid; EEG, electroencephalogram; F, female; FH, family history; HC, head circumference; Ht, height; IQ, intelligence quotient; m, male; ND, not done; Wt, weight.
2.4 Whole-exome sequencing
This research was reviewed and approved by the Scientific Committee of the Orodental Genetics Department and the Medical Research Ethics of the NRC in accordance with the “World Medical Association Declaration of Helsinki” in 1995 and written informed consents were obtained from the parents. A solo whole-exome sequencing (WES) was performed for the patient using SureSelect Human All Exome 50 Mb Kit (Agilent, Santa Clara, CA) and Illumina HiSeq2000 (Illumina, San Diego, CA).
3 RESULTS
WES revealed a previously reported pathogenic missense variant in exon 6 of the KCNK4 gene, c.730G>C (p.Ala244Pro). The identified variant was validated by Sanger sequencing and the mother was confirmed to be heterozygous for this variant (Figure 1g).
4 DISCUSSION
Extreme hypertrichosis and gingival fibromatosis are the most characteristic findings of our patients and they characterize few disorders that usually follow a dominant pattern of inheritance such as Hypertrichosis terminalis, generalized, with or without gingival hyperplasia (OMIM# 135,400) that was first described by Laurence (1857) in a Mexican woman with severe hypertrichosis, unusual facies, large ears, large mouth, everted lips, thick alveolar border of the upper jaw, thick lower gum, and deficiency of the upper front teeth. Later on, Witkop Jr. (1971) noted that the disorder could be associated with epilepsy and mental retardation. However, the disorder had no confirmed cytogenetic or molecular baseline. Other reported disorders associated with hypertrichosis and gingival fibromatosis to some extent include Gingival fibromatosis-1 (OMIM: 135300), Zimmermann–Laband syndrome 1 and 2 (OMIM: 135500 and 616455), and Cantu syndrome (MIM: 239850) which are caused by dominant mutations in SOS1, KCNH1, ATP6V1B2, and ABCC9 genes, respectively (Afifi et al., 2016; Hart et al., 2002; Kortüm et al., 2015).
On the other hand, Ramon syndrome (OMIM: 266270), Mucopolysaccharidosis type VII (OMIM: 253220), Donohue syndrome (OMIM: 246200) and Fibromatosis, gingival, with hypertrichosis and mental retardation syndrome (OMIM: 605400) are also associated with hypertrichosis and gingival hyperplasia but follow an autosomal recessive pattern. Therefore, it is really challenging to reach an accurate clinical diagnosis in patients with hypertrichosis and gingival overgrowth based only on the suspected mode of inheritance and detailed clinical description.
In our study, we used exome sequencing to unravel the genetic etiology of congenital hypertrichosis and gingival fibromatosis and found a heterozygous missense variant in KCNK4 gene (c.730G>C, p.Ala244Pro) segregating in the family. The c.730G>C (p.Ala244Pro) was detected before in one of the three patients descried by Bauer et al. (2018). In fact, we can observe a homogenous mutation pattern in KCNK4 gene as so far only two variants have been identified: the c.515C>A (p.Ala172Glu) in two unrelated patients and c.730G>C (p.Ala244Pro) in our patients and one of the patients by Bauer et al. (2018). To our knowledge, our study presents the first inherited KCNK4 variant as the three patients described by Bauer et al. (2018) were sporadic and the identified variants were all “de novo”.
The phenotype of our patient (Patient 1) was reminiscent of the three patients described by Bauer et al. (2018). They all shared the same dysmorphic facies and orodental findings. In regard to the neurological findings, they all had hypotonia and seizures with variable types. However, they showed variability in the motor and mental development and ophthalmologic findings. Normal motor development was noticed in our patient and Patient 2 by Bauer et al. (2018), whereas the remaining two patients had delayed motor development. Our patient had borderline mentality similar to Patient 2 of Bauer et al. (2018) who had low average IQ while the other two patients had more severe intellectual disability. In addition, our patients and Patient 2 of Bauer et al. (2018) had no visual impairment while Patients 1 and 3 had nystagmus and bilateral optic hypoplasia (Bauer et al., 2018). Surprisingly, Patient 2 of Bauer et al. (2018) had the same variant found in our patients. Therefore, we might speculate that the c.730G>C (p.Ala244Pro) causes a milder phenotype and is not associated with eye problems when compared to patients carrying the c.515C>A (p.Ala172Glu). This might lead us to suggest the presence of a phenotype–genotype correlation.
Brain imaging findings in patients with KCNK4 variants seem non-specific. Thin corpus callosum, enlarged ventricles, mild enlargement of bilateral frontal-insular and temporal subarachnoid spaces were noted in individual cases (Bauer et al., 2018). In addition, our patient had wide CSF space. Additional findings found in our patient were atrial fibrillation and lax joints which were not observed in previously reported patients. In contrast, one of the patients described by Bauer et al. (2018) had brachydactyly and congenital hip dysplasia.
TRAAK channels are expressed in periodontal ligament fibroblasts, KCNK4 mutations promote faster cell progression of gingival fibroblasts resulting in diffused gingival enlargement (Mariani et al., 2021; Urrego et al., 2014). In Patient 1, gingival fibromatosis prevented the proper eruption of teeth, which could be related to activation of K2P channels that may have subsequent effect on the mechanical stretch (Mariani et al., 2021). As the mother of our patient was previously subjected to a gingivectomy procedure at early age and showed very mild recurrence of the gingival enlargement along years, our dental surgical team was encouraged to remove the gingival overgrowth in the boy by electrosurgery method as described by Mariani et al. (2021). A modification on the suggested treatment plan was the use of postoperative soft acrylic guard to delay the suspected recurrence of gingival enlargement. In the mother case, the recurrence of the gingiva was slowed down by fixed prosthesis presence. The applied crowns and bridges used by Yaprak et al. (2012) prevented the progression of gingival growth. Moreover, the eruption of permanent dentitions hastens the gingival overgrowth (Gao et al., 2020).
In conclusion, gingival fibromatosis, hypertrichosis, facial dysmorphism, variable degree of intellectual disability, and epilepsy are the most common findings in patients with KCNK4 variants. Ophthalmologic abnormalities and motor delay are variable-associated findings and seem to be variant specific. In addition, we think that the cardiac arrhythmias in the form of atrial fibrillation detected in our patient are one of the sequelae to the K+ channel alteration. Surgical dental management of patients had shown tremendous success and exhibited a great impact on the patients' life and psychological stability.
AUTHOR CONTRIBUTIONS
Mohamed Abdel-Hamid, Mona Aglan, and Nehal Hassib designed and supervised the study. Rasha Elhossini was actively involved in the clinical evaluation and follow-up of the patient. Nehal Hassib, Inas Sayed, and Usama Saad Hellal were involved in the orodental examination and the dental management. Sarah A. M. Mahmoud was involved in the histopathological study. Mohamed Abdel-Hamid was involved in whole-exome sequencing, data analysis, and interpretation. The first draft of the article was written by Rasha Elhossini, Mohamed Abdel-Hamid, and Nehal Hassib. All authors had critically reviewed the article and approved the final version.
ACKNOWLEDGMENTS
Our gratitude is extended to our dear friend the late Dr. Ahmad F. Abdel-Azeem who planned and contributed in the dental surgical procedure and passed away before accomplishing the article.
FUNDING INFORMATION
This work was funded by a research grant from the Science and Technology Development Fund (STDF), Academy of Science Research and Technology, Egypt (Grant number: 33458).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




