Saliva DNA: An alternative biospecimen for single nucleotide polymorphism chromosomal microarray analysis in autism
Abstract
Chromosomal microarray analysis (CMA) is typically performed for investigation of autism using blood DNA. However, blood collection poses significant challenges for autistic children with repetitive behaviors and sensory and communication issues, often necessitating physical restraint or sedation. Noninvasive saliva collection offers an alternative, however, no published studies to date have evaluated saliva DNA for CMA in autism. Furthermore, previous reports suggest that saliva is suboptimal for detecting copy number variation. We therefore aimed to evaluate saliva DNA for single nucleotide polymorphism (SNP) CMA in autistic children. Saliva DNA from 48 probands and parents (n = 133) was obtained with a mean concentration of 141.7 ng/μL. SNP CMA was successful in 131/133 (98.5%) patients from which we correlated the size and accuracy of a copy number variant(s) called between a proband and carrier parent, and for a subgroup (n = 17 probands) who had a previous CMA using blood sample. There were no discordant copy number variant results between the proband and carrier parent, or the subgroup, however, there was an acceptable mean size difference of 0.009 and 0.07 Mb, respectively. Our findings demonstrate that saliva DNA can be an alternative for SNP CMA in autism, which avoids blood collection with significant implications for clinical practice guidelines.
1 INTRODUCTION
Autism is recognized as a spectrum of clinically and genetically heterogeneous disorders that are highly heritable. Although the genetic etiology is not completely understood a genetic cause can be found in approximately 30–40% of autistic individuals (Schaefer et al., 2013). Associated G-band karyotype abnormalities previously identified include maternally inherited duplications of the chromosome 15q11q13 “Prader Willi/Angelman syndrome” region, terminal deletions of chromosome bands 2q and 22q, and deletions within chromosome band 7q31 (Rosenfeld et al., 2010). However, the autism genomic landscape is now recognized to also involve not only single-gene disorders resulting from single nucleotide variation (SNV) or nucleotide triplet repeat expansion (Fragile X syndrome) but also copy number variation (CNV). Both Fragile X and Chromosome Microarray Analysis (CMA) are now considered standard of care and first-tier genetic tests for autism (Schaefer et al., 2013). Genetic testing for other single-gene disorders associated with autism is not routine and typically directed by co-occurring clinical or phenotypic features.
Several previous large-scale genome-wide CNV studies have consistently shown that CNV is enriched in autism vs. controls with overall detection rates estimated in unselected cohorts at approximately 10% with higher rates ranging from 11.6% to 12.5% in more specific autism cohorts up to 27.5% in syndromic autism (Rosenfeld et al., 2010; Schaefer et al., 2013). Recent consecutive case series from diagnostic clinical laboratories have reported CNV detection rates for autism between approximately 20.0 and 24.4% (Ho et al., 2016; Roberts et al., 2014).
These early studies established that CNV associated with autism is usually rare (<1% population frequency), large in size (>500 kb), and defined by recurrent “hotspots” or “CNV susceptibility loci.” Interestingly, smaller CNVs were not found enriched in autism. “Hotspot” CNV tends to overlap the critical region of the reciprocal deletion/duplication syndromes associated with developmental delay (DD)/intellectual disability (ID). The highest CNV rates involve deletions and duplications within chromosome band 16p11.2. Other recurrent CNVs include three regions within band 15q11.2q13.3 and less frequently in bands 1p36.22, 1q21.1, 7q11.23, 13q14.3, 16p13.2, 22q11.2, and 22q13.33, among others (Girirajan et al., 2013; Menashe et al., 2013; Rosenfeld et al., 2010; Sanders et al., 2011; Schaefer et al., 2013; Sebat et al., 2007). Such CNV may harbor single or multiple genes and while studies have failed to find the elusive autism-specific genes among CNV susceptibility loci, the size of the deletion or duplication has been shown to influence autism phenotype associations. As deletion size increases nonverbal IQ decreases but not for duplications. In contrast, as duplication size increases autism severity and repetitive or stereotypic behavior increase but not for deletions. This supports the hypothesis that increased gene dosage enhances autism severity while haploinsufficiency is associated more strongly with DD and ID (Girirajan et al., 2013). Most of these large-scale studies involved simplex autism families (single proband) and were found enriched for de novo rare and large (>500 kb) CNVs. In contrast, studies of multiplex families (more than one affected sibling) found that rare and large CNVs were more likely inherited with a lower rate of de novo CNV consistent with multigenic and/or polygenic risk loci (Leppa et al., 2016). Intriguingly, very few autism-specific genes were refined among all these early studies likely due in part to their low (rare) frequency, genome-wide distribution, and variable penetrance and expressivity of an associated phenotype (Girirajan et al., 2013; Menashe et al., 2013; Sanders et al., 2011; Sebat et al., 2007). This failure is consistent with the notion that an imbalance of multiple genes contributes to the autism phenotype (Girirajan et al., 2013). Research efforts continue, however, with a recent report that integrated CNV and single nucleotide variant (SNV) whole-exome data and found novel de novo CNVs as well as rare inherited CNVs, encompassing single genes that were proposed as potential candidates associated with autism: VPS13D, CNTNAPS, PRDK2, RBFOX1, WWOX, MACROD2, NCNLS, ABPBAS and CTNNA3 (Bacchelli et al., 2020).
DNA extracted from peripheral blood yields high molecular weight (HMW) DNA of high purity, which has been the gold standard for genetic testing. However, it requires invasive venipuncture and special specimen handling requirements, cold storage, and transport. Other disadvantages include avoidance of blood-draw due to needle-stick phobia, reluctance for repeat blood draws, difficult vein access, and hesitation of the family/care givers or the phlebotomist to draw blood from elderly and feeble individuals, or young children/infants. Blood sampling from autistic patients has additional challenges involving stereotypic behavioral movements, which may require patient restraint or sedation for collection. In contrast, saliva offers an alternative biospecimen for DNA that can be collected noninvasively. It contains ~4.3 × 105 cells/mL comprised of epithelial and leucocyte cell types derived from embryonal ectoderm and mesoderm, respectively. High-quality DNA can be obtained from saliva even when stored at room temperature prior to processing from 6 months to several years (Sun & Reichenberger, 2014).
Saliva has been recognized previously as a source of DNA for genetic and genomic studies using PCR-based methods, next-generation sequencing whole-exome and whole-genome studies, including genome-wide association studies (GWAS) using single nucleotide polymorphism (SNP) genotyping (Sun & Reichenberger, 2014). The majority of early GWAS studies showed no difference between DNA from saliva or blood for SNP genotyping with call rate concordance >97%. However, saliva has not been the specimen of choice for CMA and there are early controversial reports over whether saliva DNA is comparable to blood DNA for CNV detection. In one small study, saliva was as good as blood (Dellinger et al., 2010), however, another showed significant differences (Fabre et al., 2014) and yet another study showed better detection using DNA from the blood than buccal swab (Marenne et al., 2011). Very few reports have been published using saliva DNA for CMA testing within clinical diagnostic laboratories (Francis et al., 2022; Reiner et al., 2017), and as far as we are aware, there are none investigating autism cohorts.
Therefore, we performed SNP CMA using saliva DNA with the main focus being to evaluate the clinical utility of this biospecimen for identifying CNV among children with autism.
2 METHODS
-
Editorial policies and ethical considerations:
The study was approved by the Sydney Children's Hospital Network Human Research Ethics Committee, reference number: HREC/14/SCHN/269.
-
Study design/sample cohorts:
The study was undertaken between August 2018 and July 2019 and comprised autistic children (mean age 5.5 years, range 2–12 years) recruited from metropolitan Sydney and regional areas of New South Wales, Australia. Saliva was collected from a total of 48 probands and their parents of which 17 probands (mean age 5.7 years, range 2–11 years) had a previous comparative genomic hybridization (CGH) chromosome microarray analysis using a blood sample. All children had a confirmed diagnosis of Autism Spectrum Disorder as per Diagnostic and Statistical Manual of Mental Disorders-5 and there were no exclusions based on cognitive or language level or on the presence of any co-occurring conditions. All children completed assessments regarding autism features using the Autism Diagnostic Observation Schedule (Lord et al., 2012), cognitive level using the Mullen Scale of Early Learning (Mullen, 1995), and adaptive functioning abilities using the Vineland Adaptive Behavior Scale, second edition (Sparrow et al., 2005). Whole-exome or whole-genome sequencing of these children and longitudinal clinical follow-up were not part of this study's protocol.
-
Saliva DNA extraction:
Saliva was collected using the Oragene® DNA kit and DNA was extracted using the prepIT.L2P kit and resuspended in 50 μL Tris-EDTA buffer (pH 8.0), as per the manufacturer's instruction. DNA purity was assessed by 260/280 nm and 260/230 nm optical density (OD) ratios using Implen NanoPhotometer. Standard satisfactory ratios are typically 1.8 and >2.0, respectively. DNA concentration and high molecular weight (HMW) integrity were assessed by Tapestation (Agilent Technologies) and Qubit® 2.0 Fluorometer (Life Technologies).
Coefficient of Variation (CV%) for quality metrics was calculated as:
CV% = standard deviation (SD)/mean *100.
-
SNP CMA:
SNP CMA was performed using the Infinium CytoSNP-12 Beadchip v2.1 and NextSeq 550 instrument, according to the manufacturer's protocol. Recommended DNA input is 200 ng, however, we used DNA input as low as ~4–10 ng depending on saliva DNA concentration; for example, samples with ~1 ng/μL. Raw CytoSNP-12 Beadchip data were processed using KaryoStudio (Illumina) for beadchip quality metrics and then analyzed by BlueFuse Multi v4.1.3 (Illumina) using the Human Genome Reference build GRCh37. Satisfactory Beadchip quality metrics were defined as Derivative Log Ratio (DLR) ≤0.24, LogR Deviation ≤0.2, B-Allele Frequency Deviation (BAF Dev) ≤0.03, and SNP call rate ≥0.98. CNV was called when involving ≥15 consecutive SNP probes and LogR ratio at approximately −0.4 (deletion) or 0.2 (duplication). A region of homozygosity (ROH) was called when ≥2 Mb and at least 500 probes were involved. Trio or Duo genotype analysis was performed using BlueFuse Multi, as per the manufacturer's instruction. CNV mosaicism >10–20% is detectable by visual inspection of SNP probes within the BAF plot and correlating with the LogR ratio, as previously published (Conlin et al., 2010; Cross et al., 2007).
Prior to our study, the SNP CMA platform used herein had been fully validated with assay performance characteristics (sensitivity, specificity, robustness, and repeatability), limit of detection and measurement uncertainty established and deemed “fit for purpose” using DNA from Blood, which was performed in accordance with Australia's federal regulatory framework, including the Therapeutic Goods Administration (TGA) and National Pathology Accreditation Advisory Council (NPAAC) “Requirements for the development and use of in-house invitro diagnostic medical devices” (NPAAC, 2018). The SNP CMA platform comprised part of the laboratory's accreditation scope as assessed by the National Association of Testing Authorities (NATA) and found compliant with the International Standard “ISO 15189: Medical laboratories—Requirements for quality and competence.” Subsequent “verification studies” have incorporated the use of DNA from different biological sources, including bone marrow, amniotic fluid, chorionic villus, “products of conception,” CD138+ plasma cells, and fresh solid tumor tissue. We therefore consider the use of saliva DNA here a “verification study.”
CNVs were classified as benign, likely benign, pathogenic, likely pathogenic, or “variants of uncertain significance” (VoUS) according to recent guidelines (Riggs et al., 2020). Evidence-based data were gathered from the UCSC Genome Browser, Online Mendelian Inheritance in Man (OMIM), ClinGen Dosage Sensitivity Map, Database of Genomic Variants (DGV), our own “in-house” database and the Database of Chromosomal Imbalance and Phenotype in Humans using ENSEMBL Resources (DECIPHER) (Firth et al., 2009).
3 RESULTS
3.1 Saliva DNA and Beadchip data quality
A summary of quality metrics for DNA and CytoSNP-12 Beadchip for all saliva samples (n = 133) can be found in Table 1. The mean saliva DNA concentration was 141.7 ng/μL with a wide range (1–474 ng/μL), which showed mean 260/280 nm and 260/230 nm ratios of 1.76 and 1.15, respectively; noting OD ratio was not recorded for 8/133 samples. Variation in DNA concentration and OD ratios as measured by CV% was 76.3%, 13.5%, and 57.4%, respectively. Saliva DNA produced mean beadchip quality metrics for DLR, LogR Dev, BAF Dev, and call rate of 0.14, 0.16, 0.03, and 0.99. The CV% of beadchip quality metrics is shown in Table 1.
| n = 133 | CytoSNP-12 Beadchip | ||||||
|---|---|---|---|---|---|---|---|
| DNA ng/μL | 260 nm/280 nm | 260 nm/230 nm | DLR | LogR Dev | BAF Dev | Call rate | |
| Range | 1.0–474.0 | ||||||
| Mean | 141.7 | 1.76 | 1.15 | 0.14 | 0.16 | 0.03 | 0.99 |
| Median | 109.0 | 1.79 | 1.06 | 0.13 | 0.15 | 0.03 | 0.99 |
| s.d. | 108.1 | 0.24 | 0.66 | 0.043 | 0.057 | 0.007 | 0.011 |
| CV% | 76.3 | 13.5 | 57.4 | 30.2 | 35.9 | 21.5 | 1.1 |
| Test failure | 2 | ||||||
Successful SNP CMA results were obtained in 131/133 (98.5%) samples, however, two samples (Fus_023 and Fus_047) failed due to unsatisfactory Beadchip quality for all metrics (see Table S1). Fus_23 had low DNA at 1 ng/μL with poor 260/280 nm and 260/230 nm ratios of 0.87 and 0.13, respectively. Fus_047 had low DNA at 5 ng/μL with unrecorded OD ratios (see Table S1). There was insufficient DNA to assess HMW integrity for both samples.
In the subgroup of 17 probands (Families 1–17) with a previous CGH microarray, we compared saliva and blood DNA quality in a total of 22 samples (17 probands, 5 parents) (see Table 2; Table S1). The mean DNA concentration for saliva versus blood was 124.0 versus 322.4 ng/μL, which showed mean 260/280 nm and 260/230 nm ratios at 1.90 versus 1.78 and 1.45 versus 1.84, respectively. We also compared DLR quality metrics for CytoSNP Beadchip and CGH microarray, which showed mean values of 0.14 and 0.18, respectively (see Table S1). The other beadchip metrics cannot be compared. Note, the CGH array failed two blood DNA samples (sample ID 005_P and 007_P) due to DLR values being greater than 0.24.
| n = 22 | CytoSNP-12 Beadchip | ||||||
|---|---|---|---|---|---|---|---|
| DNA ng/μL | 260 nm/280 nm | 260 nm/230 nm | DLR | LogR Dev | BAF Dev | Call rate | |
| Saliva (n = 22) | |||||||
| Range | 1.0–361.0 | ||||||
| Mean | 124.0 | 1.90 | 1.45 | 0.14 | 0.16 | 0.03 | 0.99 |
| Median | 72.5.0 | 1.76 | 0.99 | 0.13 | 0.14 | 0.03 | 0.99 |
| SD | 110.6 | 0.28 | 0.27 | 0.021 | 0.024 | 0.004 | 0.005 |
| CV% | 89.1 | 14.5 | 19.0 | 15.0 | 15.5 | 12.4 | 0.5 |
| Test failure | Nil | ||||||
| Blood (n = 22) | |||||||
| Range | 1.7–1000.0 | ||||||
| Mean | 322.4 | 1.78 | 1.84 | 0.18 | |||
| Median | 290.5 | 1.81 | 1.88 | 0.15 | |||
| SD | 204.0 | 0.10 | 0.37 | 0.06 | |||
| CV% | 63.3 | 5.7 | 20.0 | 35.9 | |||
| Test failure* | 2 | ||||||
- * One sample had DNA at 1.7 ng/μL and the other 271 ng/μL but poor DNA quality resulting in unsatisfactory DLR values for the CGH microarray data.
We also assessed saliva DNA integrity in this subgroup and their parents at the end of the study, which included 42 samples (17 probands, 25 parents) with results shown in Figure S2. Most (n = 39) saliva showed HMW DNA ≥15,000 bp, however, one sample was completely degraded or had no remaining DNA (Figure S2a, lane D2), three samples showed a smear of 1200–15,000 bp (Figure S2b, lanes G1, E2, H2 and Figure S2c, lane A2), while another three showed DNA between 100 and 250 bp (Figure S2c, lanes B1, E1, F1). Despite this, all 42 samples had produced a valid SNP CMA result prior to DNA integrity assessment with satisfactory beadchip quality metrics (see Table S1). DNA integrity was not assessed for the remainder samples (n = 91) in the study.
3.2 CNV detection and size variation estimate
In the subgroup, we also compared CNV detected in saliva vs. blood DNA using CytoSNP Beadchip vs. CGH microarray platforms. All CNVs in saliva DNA were verified as concordant, and the mean size difference was 0.070 Mb or 70 kb (s.d. = 0.096); see Table S4. We next assessed the accuracy of CNVs called in saliva DNA by BlueFuse Multi between the 22 probands and parents where a CNV was inherited. All CNVs were verified as concordant and the mean difference in CNV size called by the BlueFuse Multi software was approximately 0.009 Mb or 9 kb (SD = 0.021); see Table S5.
3.3 CNV pathogenicity and burden
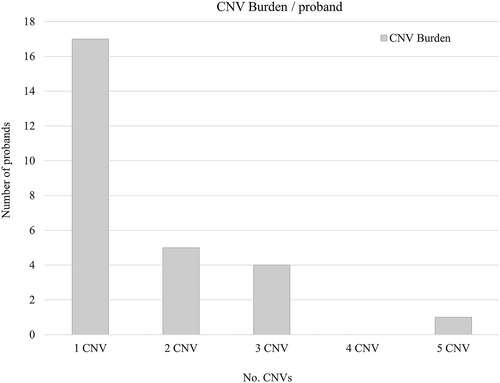
SNP CMA results for all saliva DNA samples can be found and summarized in Table S2, which comprised 48 probands and parents analyzed as either trios (n = 38), duos (n = 9), or singleton (n = 1). Trio or duo genotype analysis confirmed expected familial relationships. Excluding the two samples that failed, no CNV was found in 17/46 (37.0%) probands, however, two of these showed ROH at 4.8% and 10.4%. In 29/46 (63.0%) probands with CNV, there was a total count of 44 CNVs classified as either pathogenic (n = 3), VoUS (n = 3), likely benign (n = 13), or benign (n = 25); see Table S3. Among the 27 probands, a single CNV was found in 17 probands, two CNV in five, three CNV in four, and five CNV in one proband; see Figure 1. CNV was found inherited from a carrier parent in 23 probands.
4 DISCUSSION
DNA from blood is considered the gold standard for genetic testing, including for CMA. While saliva DNA has been extensively used with other genetic testing methods, there are surprisingly few reports of saliva DNA used for CMA testing, especially among clinical diagnostic laboratories. Herein, we have successfully explored the utility of saliva as an alternative biospecimen among a series of autistic probands.
We successfully obtained SNP CMA results from almost all saliva samples (98.5%), except for two (Fus_023 and Fus_047). Overall, mean saliva DNA concentration was 142.1 ng/μL but this varied widely (1–474 ng/μL) accompanied by an acceptable mean 260/280 nm ratio (1.76) but low mean 260/230 nm ratio (1.15) (Table 1). The variable DNA yield most likely reflects the collection technique and variable saliva volume while the low OD ratios suggest DNA impurities from residual solvent contaminants remaining after DNA extraction. Despite this, we did not further purify saliva DNA before use but still obtained valid SNP CMA results, which suggests the low 260/230 nm ratio did not significantly affect assay performance. Indeed, the mean beadchip quality metrics for DLR, LogR Dev, BAF Dev, and call rate overall saliva samples were exceptionally good (Table 1). This included several samples with unsatisfactory BAF Dev values (0.04 or 0.05) and the two failed samples (FUS_023 and FUS_047) with unacceptable DLR, LogR Dev, and BAF Dev values; see Table S1. Despite occasional unsatisfactory BAF Dev values, we could still successfully interpret results in almost all samples, except for the two that failed. However, laboratories should take care when interpreting suboptimal SNP CMA data and evaluate their own acceptable quality metrics before the use of saliva DNA.
These findings suggest that saliva DNA of variable yield and purity may still produce a successful result. This success may in part reflect the robustness of the Infinium Beadchip technology to produce high-quality beadchip data. Beadchip data quality might be further improved by developing an “in-house” saliva DNA clusterfile (.egt file) to reduce “noisy” genotyping data, which could particularly benefit samples with poor data quality. We note two sample failures (Fus_023 and Fus_047), which most likely reflected low DNA yield (concentrations at 1 ng/μL and 5 ng/μL) and/or poor DNA purity (Table S1). However, in our experience, DNA samples of <5 ng/μL may still produce an SNP CMA result, as exemplified by a saliva sample (001_M) with DNA at 1 ng/μL, although with a suboptimal BAF Dev metric. As part of the study, we also showed that duo and trio genotype analyses can be performed using saliva DNA and confirmed familial relationships in 47/48 probands, even for one of the failed samples (Fus_023). The other failed sample (FUS_047) could not be genotyped.
In a subgroup of 17 probands (and five parents) with CGH array and therefore both saliva and blood DNA, we confirmed that blood DNA has greater yield (mean 322.4 ng/μL) and purity than saliva, according to 260/280 nm (1.78) and 260/230 nm (1.84) ratios (Table 2). However, blood DNA can still result in CGH array test failure as evidenced by two samples (sample IDs 005_P and 007_P) with poor DLR values (see Table S1). Despite the lower DNA yield and purity in our saliva DNAs, we found saliva still yields DNA of sufficient quantity and purity for successful SNP CMA, which was even successful for the two probands with failed CGH arrays.
We next compared CNVs identified between saliva and blood DNA in this subgroup and all were concordant with a mean size difference ~70 kb (Table S4). The size discrepancy here reflects the different probe coverage/spacing within the CNVs and resolution of the different microarray platforms. We then looked at CNV as called by Bluefuse Multi using only saliva samples for 23 probands with an inherited CNV. We found the mean size difference of called CNV was ~9 kb (Table S5). Despite this size variation, we deemed this acceptable and attributable to the number of consecutive probes within the CNV as called by the software and background noise of the CytoSNP assay. Any size discrepancy typically reflected whether a single SNP probe was included in the CNV call, consistent with the mean backbone probe spacing of ~9 kb for the CytoSNP-12 beadchip.
In 46 probands (Tables S2 and S3) with successful saliva SNP CMA results, there were 29 probands (58.7%) that harbored a total of 44 CNVs. The majority were classified as either likely benign (n = 13) or benign (n = 25) with the remainder VoUS (n = 3) or pathogenic (n = 3). There were no likely pathogenic CNV. Of the pathogenic CNVs, two were duplications within 16p13.11, found in different probands. One showed a paternally inherited 1.26 Mb duplication (Sample ID 001_P) and the other a maternally inherited 1.16 Mb duplication (Sample ID 219_P). Both encompass the same critical region known as the recurrent “16p13.11 duplication neurocognitive disorder susceptibility” locus; see DECIPHER database. The remaining proband (sample ID 013_P) had a de novo 0.12 Mb deletion within 16p11.2, also known as the recurrent “16p11.2 deletion syndrome”; see GeneReviews NBK11167 and OMIM #611913. Of the VoUS, these reflected a CNV of unknown inheritance (sample 016_P) and two deletions in one proband that were de novo or maternally inherited (sample 236_P). Benign CNVs reflected those overlapping known population CNVs and likely benign CNVs were those inherited from an “apparently” unaffected parent; acknowledging that any potential phenotype in a carrier parent may be masked by variable expressivity and reduced penetrance.
We also assessed CNV burden among the 27 probands with CNV and found they harbored a single CNV (n = 17 probands) followed by two CNVs (n = 5), three CNVs (n = 4), and five CNVs (n = 1). Although the majority of these CNVs were classified as likely benign or benign, it is interesting that 10 probands had two or more CNVs. Increased CNV burden among individuals with neurodevelopmental phenotypes has been correlated with severity, including among autism. However, the effect size was reported greater in those with both autism and ID, rather than autism alone (Girirajan et al., 2011). Our study size is too small to draw any significance regarding CNV burden, however, it is interesting to note that one proband harbored five CNVs (Sample ID: 236_P).
Overall, the study findings as a whole demonstrate that saliva DNA is comparable to blood DNA for CNV detection. The successful and accurate identification of CNV in our autistic cohort is consistent with the reliability of previous studies. In this regard, in another ID/DD cohort of limited sample size (n = 20), saliva DNA was also shown to be reliable for successful CNV detection (Reiner et al., 2017). More recently, SNP CMA using saliva DNA has been reported to provide an additional ~4% diagnostic yield, particularly for detecting somatic mosaicism for chromosomal aneuploidy and terminal segmental abnormalities (Francis et al., 2022). Unfortunately, we did not observe mosaicism in our study but cannot completely exclude low-level mosaicism at, or below, the generally well-accepted lower detection limit (~10%) of SNP CMA (Conlin et al., 2010; Cross et al., 2007).
Limitations of our study include the relatively small sample size and missing saliva DNA OD readings for eight individuals, however, we considered this to have an insignificant effect on assessing DNA purity. Furthermore, due to limited resources, we did not compare CNV between blood vs. saliva DNA on the same CytoSNP-12 SNP CMA platform but instead verified CNV obtained from 17 probands with previous CGH array data, which we considered to be a reasonable orthogonal method of comparison. Except for these first 17 probands, we also did not verify CNVs detected in saliva DNA using another microarray platform due to resource constraints, but in 23 probands, they were verified as inherited from a parent. While the study was not designed or sufficiently powered to identify novel CNVs in autism, it provides promising results on the use of saliva for SNP CMA testing within this population.
Saliva has the advantage of being an alternative biospecimen that does not require special handling, storage and transport conditions, or immediate processing. It can be collected in a noninvasive manner using simple instructions, thereby avoiding phlebotomy and anxiety over needle-stick phobia. This is especially the case for young autistic children with sensory or behavioral difficulties that pose significant issues for blood collection, or for patients where it is challenging to perform blood collection. Saliva collections can even be done at home rather than in clinical or pathology settings, which has the added advantage that saliva collection kits can be mailed to remote/rural areas, where it can be challenging for families to visit a blood collection center. Furthermore, saliva collection can be additionally advantageous following comprehensive face-to-face clinical assessment in the event of an abnormal genetic test result that requires subsequent follow-up testing of other family members, which can be carried out by saliva kits being sent out and returned by routine mail without needing families (especially those from rural/remote areas) to make further visits to clinical facilities. Disadvantages include potential reduced DNA yield and purity and risk around improper specimen collection, labeling, and sample provenance due to self-collections. However, the risk can be reduced via education and awareness provided using online TeleHealth calls or demonstration videos with further direct supervision offered by local staff for specimen collection and labeling, if needed.
5 CONCLUSION
Our study has shown that saliva yields DNA of sufficient quantity and quality to perform SNP CMA with high test success rate and accuracy for calling CNV thereby demonstrating clinical utility as an alternative biospecimen for genetic testing in autism. More specifically, autistic individuals and in particular children with sensory and communication issues may experience significant physical and psychological discomfort from invasive blood collection. The option of saliva collection has several advantages over blood collection, which can be particularly challenging for population groups such as autistic cohorts where physical restraint or sedation of children can be avoided. Our findings have important and significant implications for the use of saliva in clinical practice guidelines for SNP CMA and genetic testing.
AUTHOR CONTRIBUTIONS
Dale C. Wright, Natalie Silove, and Valsa Eapen conceptualized and designed the study. Valsa Eapen acquired funding. Dale C. Wright wrote the first draft of the manuscript. Maria L. Baluyot, Johanna Carmichael, Artur Darmanian, Ngaire Jose, Con Ngo, Luke St Heaps, Katherine Holman, Sylvia Ziso, Feroza Khan, and Anne Masi acquired the data. All authors contributed to the review, editing, and approval of the final manuscript.
ACKNOWLEDGMENTS
We wish to thank all the children and their families who participated in this study and also acknowledge the staff within the Cytogenetics and Nucleic Acid Unit, Sydney Genome Diagnostics, and The Children's Hospital at Westmead who supported this work. Some of the data used in this project were provided by Autism CRC from the Australian Autism Biobank, with appropriate ethics approval. The authors gratefully acknowledge the Australian Autism Biobank participants and their families, the Australian Autism Biobank Team for cohort coordination and data collection (Andrew Whitehouse, Dora Abbondanza, Gail Alvares, Erin Beattie, Jolene Berry, Vandhana Bharti, Grace Christou, Dominique Cleary, Paul A Dawson, Melanie De Jong, Cheryl Dissanayake, Kendra Dommisse, Valsamma Eapen, Mira Frenk, Jacob Gratten, Rachel Grove, Claire Hafekost, Maryam Haghiran, Alexis Harun, Nicole Hayes, Anjali Henders, Honey Heussler, Helen Holdsworth, Anneliese Hopkins, Anna Hunt, Rachel Jellett, Feroza Khan, Lauren Lawson, Deborah Lennon, Jodie Leslie, Anne Masi, Nisha Mathew, Tiana McLaren, Candice Michael, Melanie Muniandy, Melissa Neylan, Michaela Nothard, Brooke Peden, Mridu Radhakrishnan, Ola Rajapakse, Emma Raymond, Felicity Rose, Natalie Silove, Ashley Thomson, Leanne Wallace and Naomi Wray), and the Australian Autism Biobank project contributing institutions (University of Western Australia, Sydney Children's Hospital Network, Telethon Kids Institute, University of New South Wales, La Trobe University, Mater Medical Research Institute, Institute for Molecular Biosciences: University of Queensland, Wesley Medical Research and PathWest). Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
This work was supported by the Autism CRC Ltd. (grant/award no.: 1.059RS).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.




