The mitochondrial tRNA MT-TW m.5537_5538insT variant presents with significant intra-familial clinical variability
Abstract
Mitochondrial disorders can present with a wide range of clinical and biochemical phenotypes. Mitochondrial DNA variants may be influenced by factors such as degree of heteroplasmy and tissue distribution. We present a four-generation family in which 10 individuals carry a pathogenic mitochondrial variant (m.5537_5538insT, MT-TW gene) with differing levels of heteroplasmy and clinical features. This genetic variant has been documented in two prior reports, both in individuals with Leigh syndrome. In the current family, three individuals have severe mitochondrial symptoms including Leigh syndrome (patient 1, 100% in blood), MELAS (patient 2, 97% heteroplasmy in muscle), and MELAS-like syndrome (patient 3, 50% heteroplasmy in blood and 100% in urine). Two individuals have mild mitochondrial symptoms (patient 4, 50% in blood and 67% in urine and patient 5, 50% heteroplasmy in blood and 30% in urine). We observe that this variant is associated with multiple mitochondrial presentations and phenotypes, including MELAS syndrome for which this variant has not previously been reported. We also demonstrate that the level of heteroplasmy of the mitochondrial DNA variant correlates with the severity of clinical presentation; however, not with the specific mitochondrial syndrome.
1 INTRODUCTION
Mitochondrial disorders are a heterogenous group of conditions that can present with a wide range of clinical and biochemical phenotypes. These disorders tend to disproportionally affect tissues and organs with high energy demands, such as the brain, heart, skeletal muscle, and retina (DiMauro & Schon, 2003). Mitochondrial disorders can arise from pathogenic variants in either the nuclear or mitochondrial DNA (mtDNA; Gusic & Prokisch, 2021). Disorders due to mtDNA variants are maternally inherited and are influenced by heteroplasmy, threshold effect, and tissue distribution of mtDNA variants, among other factors (Baertling et al., 2014; Lake et al., 2015; Rossignol et al., 2003). Pathogenic variants may only lead to clinical disease once a threshold heteroplasmy has been reached in a cell or tissue type; therefore, the same variant may have different effects between various organs and tissues and may result in different clinical phenotypes between individuals (Baertling et al., 2014; Rossignol et al., 2003).
Leigh syndrome, or subacute necrotising encephalomyelopathy, is a common pediatric-onset mitochondrial condition. Symptoms include developmental delay or regression, hypotonia, seizures, brainstem dysfunction (e.g. swallowing or respiratory dysfunction), ataxia, and ocular abnormalities (e.g. optic atrophy, nystagmus, ophthalmoplegia; Bakare et al., 2021; Chang et al., 2020). Most frequently, Leigh syndrome presents as a developmental delay or plateau in early infancy, followed by rapid neurodegeneration, and typically results in death in early childhood. Many genetic variants have been identified to cause Leigh syndrome (Bakare et al., 2021; Lake et al., 2016; Rahman et al., 2017). The majority of cases present under 2-years-old and disease course may last from months to years (Ogawa et al., 2020; Sofou et al., 2014; Stenton et al., 2022), with recent research identifying variant-specific clinical course and prognosis (Stenton et al., 2022). Individuals often have elevated lactate in serum and cerebrospinal fluid (CSF) and symmetric T2 hyperintensities in basal ganglia, thalami, and brainstem on magnetic resonance imaging (MRI; Chang et al., 2020; Hong et al., 2020; Lee et al., 2016; Rahman et al., 2017; Sofou et al., 2014).
Another common mitochondrial phenotype is mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes, or MELAS syndrome. MELAS syndrome is characterized by stroke-like episodes that do not respect vascular territories, encephalopathy with seizures and/or dementia, mitochondrial myopathy, lactic acidosis, recurrent headaches, and vomiting. It can present at any age; although, typically presents between ages 2 to 40-years-old (El-Hattab et al., 2018; Hirano et al., 1992; Tetsuka et al., 2021). Individuals often have lactic acidosis and ragged-red fibers on muscle biopsy. MELAS is associated with the MT-TL1 m.3243A > G variant in >80% of cases, but other mtDNA variants have also been reported (El-Hattab et al., 2018; Hirano et al., 1992; Tetsuka et al., 2021).
Other mitochondrial disorders may have non-specific symptoms, including hearing loss, visual impairment, short stature, failure to thrive, and diabetes (Chi, 2015; Debray et al., 2008).
We present a four-generation family (Figure 1) in which multiple members carry a rare mitochondrial variant (m.5537_5538insT, MT-TW gene) with differing degrees of heteroplasmy. The pedigree demonstrates variation in degree of heteroplasmy in different tissues and presence/severity of clinical manifestations from asymptomatic to mild mitochondrial symptoms to classic MELAS and Leigh syndrome.

2 CASE PRESENTATIONS
We present a four-generation pedigree with 10 individuals tested for a familial mtDNA variant. One individual (patient 1) was found to have Leigh syndrome. One individual (patient 2) was found to have MELAS syndrome. One individual (patient 3) was found to have numerous mitochondrial features suggestive of MELAS syndrome; although, did not meet criteria for a specific syndrome. Two individuals (patients 4 and 5) have mild symptoms suggestive of a mitochondrial disorder.
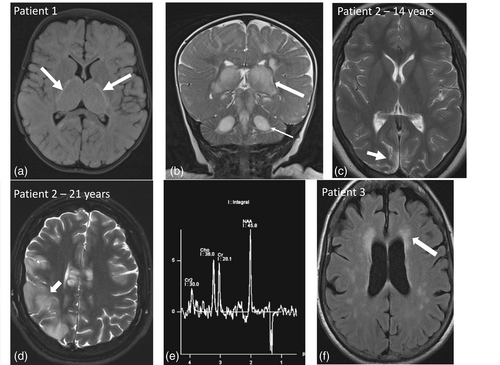
Patient 1 (individual IV-4)—index case. Patient 1 presented at 7-months-old with a 1-month history of high-frequency, low-amplitude “no-no” tremor of the head, decreased head control, and motor regression. The patient also had a history of developmental dysplasia of the hip, axial hypotonia, global developmental delay, and poor growth. Her birth weight was at the 95th percentile and had fallen to the 31st percentile at presentation. On exam, she had bilateral frontal bossing and decreased axial tone. A brain MRI was suggestive of Leigh syndrome (Figure 2a, b). Her bloodwork was in keeping with mitochondrial dysfunction (Table 1). MtDNA sequencing on blood leukocytes showed the m.5537_5538insT variant to be homoplasmic. She was started on coenzyme Q10 and thiamine supplementation.
| Person | Heteroplasmy | Phenotype | Clinical features | Neuroimaging | Labs | Treatment (max. dose) |
|---|---|---|---|---|---|---|
| I-2 (Patient 5) | Urine—30% Blood—50% |
N/A | Transient ischemic attacks, concentric left ventricular remodeling of the heart | CT Head (80 years): Chronic lacunar infarct in the right putamen, chronic subcortical microangiopathic changes | Lactate, PAA, UOA, ACP: normal | N/A |
| GDF-15: 1768 pg/mL (ref <750) | ||||||
II-1 (Patient 3) |
Blood—50%, | MELAS-like | Short stature, poor weight gain, renal impairment, optic neuropathy, easy fatiguability, myoclonus, tremor, gait instability, diffuse pain, moderate concentric LVH in her early 50s | MRI Head (48 years): few small well-defined foci on increased T2/FLAIR in periventricular white matter (most likely microangiopathic), normal MRS. MRI (52 years, Figure 2f): Mild volume loss of the brain parenchyma, mild T2 increased signal in periventricular WM, small infarcts in corona radiata with restricted diffusion, normal MRS. | Lactate, UOA, ACP: normal | Coenzyme Q10 (200 mg TID), creatine (5 g daily), taurine (10 mg daily, not tolerated), alpha-lipoic acid (200 mg BID, not tolerated), vitamin B50 complex (1 tablet daily) |
| Urine—100% | PAA: alanine at upper limit of normal | |||||
| GDF-15: 2123 pg/mL | ||||||
| Electroretinal studies (50 years): normal | ||||||
| II-2 | Blood—20% | N/A | Asymptomatic | N/A | Lactate, PAA, UOA, ACP: normal | |
| Urine—27% | GDF-15: 681 pg/mL | |||||
III-1 (Patient 2) |
Muscle—97% | MELAS | Presented with breath-holding spells at 3 months of age and febrile seizures. Many different seizure types, including epilepsia partialis continua. She had normal early development but had regression resulting in intellectual disability. She also developed microcephaly, diffuse weakness, wheelchair dependence, memory loss, progressive visual impairment with optic atrophy, migraine headaches, and concentric LVH. Deceased at 21 years | MRI (4 years): Normal | Lactate (blood): 2.3–4.5 mmol/L | For seizures (throughout her lifespan): Phenytoin, clobazam, clonazepam, valproic acid, topiramate, carbamazepine, lamotrigine, piracetam, levetiracetam, zonisamide, primidone |
| MRI (8 years, in status epilepticus): Increased T2 and FLAIR in left thalamus | Lactate (CSF): 2.4 mmol/L | |||||
| PAA: alanine mildly elevated | ||||||
ACP: normal UOA: normal |
||||||
MRI (9.5 years): Hyperintense T2 signal in right occipital and posterior right parietal, lactate peak in right occipital area. MRI (14 years, Figure 2c): Multiple areas of gliosis related to remote injury. Basal ganglia and thalami were spared. No diffusion restriction to suggest acute changes. |
Brain biopsy: unremarkable mitochondria, rarefaction of cortex, minimal lymphocytic inflammation of white matter and more prominent microglial and macrophage infiltration, rare dystrophic axons, and some neurons with “tangle-like” structures. |
|||||
| MRI (21 years, time of death, Figure 2d and 2e): Multiple areas of infarction in different vascular territories, including in body of corpus callosum, right frontal lobe, right parietal lobe, and right lentiform nucleus. Lactate peak in corpus callosum. | Muscle biopsy: mildly reduced activity of complexes I, II, III, and IV with normal muscle fiber shape and size, no ragged red fibers or COX-negative fibers, no glycogen or lipid deposition, and mild patchy areas of decreased SDH and NADH staining. |
|||||
Endomyocaridal biopsy: non-specific findings of myocarditis. |
||||||
| NCS, VEP, electroretinal studies: normal | ||||||
| III-2 | Blood—20% | N/A | Asymptomatic | N/A | Lactate: normal | N/A |
| Urine—40% | ||||||
III-3 (Patient 4) |
Blood—50% | N/A | Mild–moderate sensorineural hearing loss | MRI (31 years): Brain MRI within normal limits. No evidence of mitochondrial disorder or remote ischemia. Normal MRS. | Lactate, PAA, UOA, ACP: normal GDF-15: 492 pg/mL |
Coenzyme Q10 (100 mg BID), creatine (5 g daily), alpha-lipoic acid (200 mg daily) |
| Urine—67% | ||||||
| III-5 | Blood—49%, | N/A | Asymptomatic | N/A | Lactate, PAA, UOA, ACP: normal GDF-15: 315 pg/mL |
N/A |
| Urine—64% | ||||||
| IV-1 | Blood—40% | N/A | Asymptomatic | N/A | Lactate: normal | N/A |
| IV-2 | Blood—20% | N/A | Mild developmental delay due to second genetic diagnosis | N/A | Lactate: normal | N/A |
IV-4 (Patient 1) |
Blood—100% | Leigh Syndrome |
Presented at 7 months of age with a head tremor, global developmental delay with motor regression, axial hypotonia, and poor growth. EEG was normal and brain MRI was suggestive of Leigh syndrome. Over months, she had clinical deterioration, including feeding issues requiring gastrostomy tube insertion, irritability, worsening hypotonia, dystonia, and seizures. Ongoing significant deterioration in function, refractory seizures, dystonia, and irritability | MRI (7 months, Figure 2a and 2b): Multiple areas of T2 hyperintensity and T1 hypointensity with edema involving the bilateral thalami, mesencephalon, pons, central portion of the cerebellum, bilateral cerebral and middle cerebellar peduncles and bilateral mesial temporal regions and hippocampi. Diffusion restriction in corticospinal tracts and optic tracts. |
Lactate: 3.1–8.1 mmol/L (associated with compensated metabolic acidosis) | Coenzyme Q10 (25 mg BID), thiamine (50 mg TID) |
PAA: elevated alanine (730) ACP: normal |
For seizures: topiramate, vigabatrin |
|||||
UOA: increased excretion of Krebs cycle intermediates (pyruvic, succinic, fumaric, 2-ketoglutaric and citric acid) suggestive of mitochondrial dysfunction |
For irritability: clonidine, gabapentin, morphine For dystonia: clonazepam |
|||||
| CT (12 months): Interval progression of abnormal signals above with diffuse edema and hypodensity of corpus callosum. | EEG: 7 months: normal. 11 months, 16 months, 22 months: hypsarrhythmia ± focal seizures and interictal epileptiform discharges |
|||||
| GDF-15: not measured |
- Abbreviations: ACP, acylcarnitine; BID, twice daily; CSF, cerebrospinal fluid; COX, cytochrome oxidase; CT, computerized tomography; EEG, electroencephalogram; GDF-15, growth/differentiation factor-15; LVH, left ventricular hypertrophy; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; MRI, magnetic resonance imaging; NADH, nicotinamide adenine dinucleotide; N/A, not applicable; NCS, nerve conduction studies; PAA, plasma amino acids; PRN, as needed; QHS, at nighttime; SDH, succinic dehydrogenase; TID, three times daily; UOA, urine organic acids; VEP, visual evoked potentials.
Over the next few months, she had clinical deterioration including feeding issues requiring gastrostomy tube insertion, irritability, worsening hypotonia, dystonia, and seizures. Her seizure semiologies included infantile spasms and focal seizures with: (i) behavioral arrest and staring; (ii) left upper extremity tonic movements with or without progression to bilateral upper extremity tonic movement; (iii) automatisms of the mouth such as chewing and lip smacking; and (iv) apneas. She also developed episodes of left-sided facial droop with vomiting which were felt to represent “negative” type seizures. At her last visit, she was 19-months-old with significant deterioration in function, refractory seizures, difficult-to-treat dystonia, and irritability.
Patient 2 (individual III-1). Patient 2 presented with breath-holding spells at 3-months-old. At 8-months-old, she started having focal febrile seizures. She had a normal head CT scan and electroencephalogram, elevated alanine on plasma amino acid (PAA) profile, and elevated blood lactate. Seizures resolved from 2- to 4-years-old and then recurred; semiology was described as focal seizures alternating between both the right and left sides at different times. At 4-years-old, she also had possible absence seizures and visual hallucinations. MRI at that time was normal (no spectroscopy was done). At 8-years-old, she was admitted to hospital for generalized tonic–clonic status epilepticus, followed by ongoing left-sided myoclonic seizures, consistent with epilepsia partialis continua (EPC). MRI brain showed increased T2/FLAIR signal in the bilateral thalami. Blood lactate was mildly elevated, urine organic acid (UOA) profile was normal, and there was elevated alanine and glutamine on PAA. CSF lactate was mildly elevated. Retinal exam was normal. She was admitted again at 11-years-old with multifocal seizures and EPC. Brain biopsy was abnormal but not suggestive of a specific diagnosis (Table 1). She developed diffuse weakness and wheelchair dependence after this admission.
She had generally normal early development apart from some mild speech delays. With the onset of seizures, she started to show regression of motor skills. Neuropsychology testing at 6-years-old showed average verbal and nonverbal abilities. Repeat testing at 8-years-old showed she had a verbal intelligence quotient (IQ) at the 23rd percentile and nonverbal IQ at the 2nd percentile. Neuropsychological testing at 18-years-old showed mild intellectual disability with cognitive functioning at the 0.1st percentile. She also developed multisystemic medical issues as she aged including mood swings, memory difficulties, “clumsiness,” progressive visual impairment, optic atrophy, scoliosis, left spastic hemiplegia, recurrent migraine-like headaches, and concentric left ventricular hypertrophic cardiomyopathy.
Over of her life, she had many diagnostic investigations, including karyotype (46,XX), chromosomal microarray (7p22.2 microduplication of unknown significance, containing only the SDK1 gene, maternally inherited), mtDNA point mutation panel and deletion analysis, SCN1A gene sequencing, neuronal ceroid lipofuscinosis gene panel, mitochondrial complex IV gene panel, and research whole-exome sequencing. Skin biopsy was nondiagnostic due to insufficient samples. Muscle biopsy did not show ragged red fibers or cytochrome oxidase (COX)-negative fibers but did have other mitochondrial changes such as reduced activity of complexes I-IV and decreased succinic dehydrogenase and nicotinadmide adenine dinucleotide staining (Table 1). MRI at 14-years-old showed multiple areas of gliosis (Figure 2c) but no diffusion restriction to suggest acute changes.
She had a marked deterioration in her teenage years and had a metabolic stroke resulting in her death at 21-years-old. MRI showed multiple areas of infarction in different vascular territories (Figure 2d). MRI spectroscopy (MRS) demonstrated a lactate peak within the body of the corpus callosum and increased choline peak in right parietal subcortical white matter (Figure 2e). She was diagnosed at the time with a “MELAS-like syndrome;” although, she does meet the clinical criteria for MELAS as per criteria proposed by Yatsuga et al. (2012). Her autopsy showed an absence of COX staining in muscle. Post-mortem mtDNA sequencing showed the m.5537_5538insT variant at near homoplasmy (~97%) in muscle.
Patient 3 (individual II-1). The mother of patient 2 was diagnosed after the death of her daughter. She has classic features of a mitochondrial disorder, although does not fit the specific diagnostic criteria for MELAS or other specific mitochondrial disorders. We have called her presentation “MELAS-like.” Her clinical phenotype includes small size, easy fatiguability, myoclonus, tremor, optic neuropathy chronic renal impairment, gait instability, chronic pain and peripheral neuropathy, frequent headaches, moderate left ventricular concentric cardiac hypertrophy, and progressive white matter T2-hyperintense lesions on brain MRI with normal MRS (Figure 2f). She has mild memory/cognitive difficulties and MOCA at 50-years-old was 24/30. She reported difficulties with visual acuity and central visual blurring, which improved with use of filter glasses. Ophthalmological testing at 52-years-old showed optic atrophy and central scotomas consistent with mitochondrial optic neuropathy. She also had 10 miscarriages with no clear cause identified. She was found to have m.5537_5538insT variant at homoplasmic (100%) levels in urine and 50% in blood.
Patient 4 (individual III-3). The mother of patient 1 was found to have mild–moderate hearing loss. Blood heteroplasmy levels were 50% and urine level was 67%.
Patient 5 (individual I-2). The matriarch of the family has a history of transient ischemic attacks and concentric left ventricular remodeling on echocardiogram but is otherwise generally healthy in her 80s. Blood heteroplasmy level was 50% and urine level was 30%.
Other family members. Samples were collected from other family members which showed various levels m.5537_5538insT variant heteroplasmy (summarized in Figure 1 and Table 1). Full medical records of all family members were not available for review. To the best of our knowledge, no other family members have shown signs of mitochondrial disease at the time of publication.
3 DISCUSSION
This paper presents a family with the m.5537_5538insT variant in the MT-TW gene presenting with a spectrum of mitochondrial phenotypes. In this family, the degree of heteroplasmy appears associated with the severity of the clinical presentation but not with the specific mitochondrial phenotype.
In addition to differing clinical features, there are differences in the imaging phenotypes in this family. Patient 1 presented primarily with central changes involving the brainstem, thalami, and white matter with cortical sparing, in keeping with Leigh syndrome. In contrast, patient 2 presented with lesions involving the cortex and subcortical white matter in a nonvascular distribution, abnormal signal in the putamen, and ischemic changes in the corpus callosum in the acute disease phase, in keeping with MELAS.
The m.5537_5538insT variant has been reported in two families in the medical literature, both in individuals diagnosed with Leigh syndrome. It was first described by Santorelli et al. in a family with one child who died of Leigh syndrome and one child with a progressive neurologic disorder. In this family, the mutation was near homoplasmic in the proband and his brother (>92%) and lower levels of heteroplasmy were seen in four maternal relatives (42%–89%), three of whom had short stature, migraine-like headaches, and/or neuropsychiatric disturbances such as depression and psychosis (Santorelli et al., 1997).
Tulinius et al. later reported a boy with Leigh syndrome who presented with irritability and hypotonia in infancy whose symptoms progressed to nystagmus, hypertonia, optic atrophy, refractory seizures, and progressive neurodegeneration. This patient was also found to have marked complex I deficiency and COX deficiency in muscle cells. He had near homoplasmy (>95%) of the variant in blood, liver, and muscle tissues. The variant was found at 81% heteroplasmy in the blood of proband's mother who was reportedly asymptomatic. The mother's sister had two children with similar phenotypes to the proband who were both diagnosed with Leigh syndrome; although, formal genetic testing was not completed (Tulinius et al., 2003).
Prior research has also described that different mitochondrial phenotypes resulting from the same genetic variant can be explained by different levels of heteroplasmy. The mitochondrial m.8993T>G variant in the MT-ATP6 gene, when present at levels of 70%–90% heteroplasmy, may present with neuropathy, ataxia, and retinitis pigmentosa syndrome. Heteroplasmy of 90% or higher may present with a classic Leigh syndrome phenotype (White et al., 1999). Another study examining variants in MT-TK found that relatively lower levels of heteroplasmy presented as adult-onset Myoclonic Epilepsy with Ragged Red Fibers and higher levels of heteroplasmy presented as Leigh syndrome (Shtilbans et al., 2000). The current study adds to this literature by demonstrating that high levels of heteroplasmy of the same genetic variant may present with different, well-defined mitochondrial disorders.
4 CONCLUSION
This paper presents a family with the m.5537_5538insT variant in the MT-TW gene, presenting as a spectrum of mitochondrial phenotypes including Leigh syndrome, MELAS syndrome, and mild mitochondria-related symptoms (e.g. hearing loss). In individuals severely affected, the variant is near homoplasmic and lower levels of heteroplasmy were found in mildly affected and asymptomatic family members. The clinical variability in the family presented here is consistent with prior reports of variability in the severity of clinical symptoms based on the level of heteroplasmy of this variant. This family expands the phenotypic spectrum of this variant from Leigh syndrome to also include MELAS syndrome, which has not been previously reported in the medical literature. The current study also adds to the literature by demonstrating that high levels of heteroplasmy of the same genetic variant may present with different, well-defined mitochondrial disorders.
AUTHOR CONTRIBUTIONS
Lauren Strasser and Danielle Bourque performed clinical and chart review, created the figures and tables, and wrote the first draft of the manuscript. Jorge Davila provided the neuroimaging and the accompanying interpretations. Asif Doja and Pranesh Chakraborty provided clinical details. All authors provided clinical input and edited the manuscript.
ACKNOWLEDGMENTS
We thank the family for their participation in this project.
FUNDING INFORMATION
There is no associated funding for this project.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




