Characteristics of hearing impairment in patients with trisomy 18
Shoko Tamaki and Sota Iwatani contributed equally to this work.
Trisomy 18 is the second most common autosomal aneuploidy, with an estimated frequency of 1.07 per 10,000 live births (Goel et al., 2019). Most patients with trisomy 18 have a variety of systemic organ complications, including congenital heart disease, gastrointestinal disease, urologic disease, and central nervous system disease, which lead to high mortality and morbidity (Embleton et al., 1996; Rasmussen et al., 2003; Springett et al., 2015). In recent years, however, survival outcomes for patients with trisomy 18 have been changing with improvements in intensive care, including surgical intervention (Nelson et al., 2012; Nelson et al., 2016). As a result, there are an increasing number of long-term survivors with trisomy 18 (Tamaki et al., 2022). However, long-term survivors with trisomy 18 have severe developmental delays (Bruns, 2015).
Hearing is one of the most important factors in intellectual development, especially in communication abilities. Universal newborn hearing screening is recommended to identify children with congenital hearing impairment and to provide early intervention (Nelson et al., 2008). Patients with chromosomal disorders are at high risk for hearing impairment (Lieu et al., 2020; Tedeschi et al., 2015); however, patients with trisomy 18 often have difficulty with hearing screening due to their unstable general conditions such as respiratory and cardiac failure. To date, little is known about the prevalence, severity, and type of hearing impairment (sensorineural, conductive, or mixed) of patients with trisomy 18. In this study, we aimed to assess hearing screening and audiological evaluation in patients with trisomy 18 over a 7-year period.
This was a single-center, retrospective cohort study that included patients with trisomy 18 admitted to a neonatal intensive care unit (NICU) at Hyogo prefectural Kobe Children's Hospital (HPKCH) between January 2016 and December 2022. Ethical approval was granted by the Institutional Review Board of HPKCH (R4-44) with a waiver of written informed consent. Information about this study was available via the hospital bulletin board and website.
After the patients' condition stabilized for discharge, audiological evaluation was performed by attending physicians. Patients with tracheostomies required manual ventilation. Automated auditory brainstem response (AABR) testing using ALGO 5 Newborn Hearing Screener (Natus Medical Inc., CA) was performed as the first hearing screening for all patients. The stimulus intensity level was set at a 35 decibel hearing level (dB HL). Patients who failed AABR hearing screening underwent otolaryngological examination, imaging evaluation with temporal bone computed tomography (CT), and electrophysiological testing such as auditory brainstem response (ABR) and automated steady state response (ASSR). ABR and ASSR testing were performed using Neuropack MEB-2036 (Nihon Kohden, Tokyo, Japan) or Eclipse (Interacoustics, Assens, Denmark) on patients sedated with triclofos sodium. The severity of the hearing impairment was estimated as normal (<20 dB), mild (20–34 dB), moderate (35–49 dB), moderately severe (50–64), severe (65–79 dB), and profound (≥ 80 dB) based on the ABR and/or ASSR thresholds in the better hearing ear (Stevens, 2013). To distinguish between sensorineural, conductive, and mixed hearing impairments, ASSRs were elicited to air- and bone-conducted stimuli. Air conduction thresholds were measured at 500, 1000, 2000, and 4000 Hz, and bone conduction thresholds at 1000, 2000, and 4000 Hz.
Patients' data are presented as median (range) or number (%). For comparisons of patient characteristics, Wilcoxon rank-sum test was used for continuous variables and Fisher's exact test for categorical variables. p-values <0.05 were considered significant. All analyses were performed using JMP statistical software (JMP 13.0.0, SAS Institute. Inc., NC, USA).
During the study period, 40 patients with trisomy 18 were admitted to our NICU. Of these, 22 underwent hearing screening with AABR. The other 18 patients did not undergo hearing screening because of their unstable general condition. Table 1 shows a comparison of the characteristics of the patients who underwent hearing screening and those who did not. The survival to discharge rate for patients who underwent hearing screening was 91%, which was significantly higher than 28% for those who did not. The first AABR testing was performed at 79 (42–296) days of age, and 19 patients (86%) failed screening.
| All n = 40 | Hearing screening | p-value | ||
|---|---|---|---|---|
| + | − | |||
| n = 22 | n = 18 | |||
| Gestational age (weeks) | 36.4 ± 3.0 | 37.4 ± 2.1 | 35.2 ± 3.5 | 0.021 |
| Birth weight (g) | 1569 ± 404 | 1735 ± 335 | 1366 ± 396 | 0.005 |
| Female | 30 (75) | 18 (82) | 12 (67) | 0.300 |
| Apgar score at 5 min <7 | 21 (53) | 11 (50) | 10 (56) | 0.761 |
| Intubation at delivery room | 21 (53) | 11 (50) | 10 (56) | 0.761 |
| Congenital heart disease | 39 (98) | 21 (95) | 18 (100) | 1.000 |
| Esophageal atresia | 15 (38) | 5 (23) | 10 (56) | 0.050 |
| Surgical intervention | 37 (93) | 22 (100) | 15 (83) | 0.083 |
| Tracheostomy | 13 (33) | 7 (32) | 6 (33) | 1.000 |
| Survival to discharge | 25 (63) | 20 (91) | 5 (28) | < 0.001 |
- Note: Data are presented as the mean ± SD or n (%).
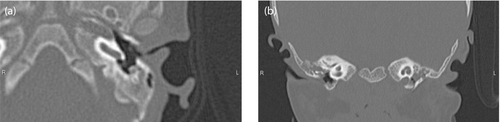
Of the 19 patients who failed screening, 14 subsequently underwent otolaryngological examination and electrophysiological testing for further audiological evaluation. All of them were noted to have external auditory canal atresia or stenosis on otolaryngological examination, of which nine were confirmed by temporal bone CT (Figure 1). ABR and ASSR testing were performed at 311 (111–924) and 266 (201–1177) days of age, respectively. The results of the ABR and ASSR testing are shown in Table 2. The severity of hearing impairment was moderate (n = 1), moderately severe (n = 7), severe (n = 3), and profound (n = 3). Ten patients for whom bone conduction thresholds were obtained were all diagnosed with mixed hearing impairment, but none had only severe sensorineural hearing impairment. Of the 14 patients diagnosed with hearing impairment by ABR and/or ASSR testing, eight initiated interventions with hearing aids at 1.6 (0.8–3.4) years of age. Four patients with hearing aids showed improvement in their response to environmental sounds by parent interviews.
| Case | Severity of hearing loss | ABR testing | ASSR testing | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Thresholds (dB) | Age at testing (days) | Air conduction thresholds at 500/1000/2000/4000 Hz (dB) | Bone conduction thresholds at 1000/2000/4000 Hz (dB) | Age at testing (days) | |||||
| Right | Left | Right | Left | Right | Left | ||||
| 1 | Profound | 100 | 100S.O. | 572 | 100S.O./100/100/100 | 100/100S.O./100S.O./100S.O. | 30/50/50 | - | 572 |
| 2 | Profound | 90 | 105S.O. | 676 | - | - | - | - | - |
| 3 | Profound | 90 | 80 | 111 | 90/100/90/80 | 100/90/80/100 | 40/40/40 | 30/55/50 | 258 |
| 4 | Severe | 70 | 90 | 484 | 80/70/50/60 | 100S.O./60/90/90 | 20/30/30 | 30/50/40 | 484 |
| 5 | Severe | 70 | 90 | 411 | 80/80/80/80 | 90/90/90/90 | 30/40/40 | 30/50/30 | 208 |
| 6 | Severe | - | - | - | 50/90/70/70 | 90/90/60/70 | 30/40/30 | 30/40/30 | 1177 |
| 7 | Moderately severe | 60 | 60 | 311 | 60/50/40/50 | 60/50/40/40 | - | - | 318 |
| 8 | Moderately severe | 60 | 70 | 231 | 80/80/70/70 | 80/70/80/80 | 10/30/20 | 10/50/50 | 231 |
| 9 | Moderately severe | 60 | 80 | 266 | 60/50/50/50 | 100/90/90/90 | 30/40/40 | - | 266 |
| 10 | Moderately severe | 60 | 80 | 201 | 90/80/60/60 | 80/70/70/60 | 30/30/20 | - | 201 |
| 11 | Moderately severe | 60 | 60 | 817 | - | - | - | - | - |
| 12 | Moderately severe | 50 | 50 | 924 | - | - | - | - | - |
| 13 | Moderately severe | - | - | - | 50/70/50/60 | 70/60/60/70 | 30/50/30 | 40/55/40 | 616 |
| 14 | Moderate | 60 | 40 | 202 | 70/60/50/50 | 40/40/40/40 | 20/30/40 | 10/20/40 | 202 |
- Abbreviation: S.O., scale out.
This is the first report to focus on the prevalence and characteristics of hearing impairment in patients with trisomy 18. Approximately half of patients with trisomy 18 are reported to have hearing abnormalities (Kepple et al., 2021); however, the prevalence and characteristics of hearing impairment have not been reported. Our cohort study found that 86% (19/22) of patients with trisomy 18 failed AABR screening. Moreover, 14 patients who underwent ABR and/or ASSR testing were all diagnosed with mixed hearing impairment of moderate or greater severity. The severity of sensorineural hearing impairment was assessed as mild to moderate. Notably, otolaryngological examination and/or temporal bone CT revealed external auditory canal atresia or stenosis in all cases. These structural abnormalities of the external auditory canal are likely to be frequently encountered complications in patients with trisomy 18, which is consistent with previous reports (Benson et al., 2023; Cereda & Carey, 2012); thus, conductive hearing impairment was considered as the primary cause of their hearing impairment. Furthermore, our study indicates that hearing aids may be effective for improving hearing function in patients with trisomy 18. Although several studies have reported that the social and communication skills of patients with trisomy 18 develop throughout their lives, they did not report about hearing impairment (Bruns, 2015; Kosho et al., 2013). Our findings suggest that hearing aid use may contribute to further development of communication skills in some patients with trisomy 18.
This study is limited by its retrospective nature and the limited number of patients who could receive audiological evaluation under stable general conditions. In addition, subjective tests, such as pure tone audiometry, were not performed because of the cognitive impairment of patients with trisomy 18. Furthermore, each hearing test was performed later than recommended. Despite these limitations, our findings offer insights for healthcare providers and families caring for a patient with trisomy 18.
In conclusion, hearing impairment is common in patients with trisomy 18, which is likely primarily due to external auditory canal atresia or stenosis. Further studies are needed to evaluate the effects of hearing aid use along with appropriate audiological evaluations on the communication skills and quality of life of patients with trisomy 18.
AUTHOR CONTRIBUTIONS
Shoko Tamaki and Sota Iwatani: Conceptualized the research project and drafted the initial letter. Sayaka Katsunuma and Masahide Otsu: Contributed to the acquisition of the clinical data. Seiji Yoshimoto: Coordinated and supervised data collection, and critically reviewed the letter.
ACKNOWLEDGMENTS
We thank Drs. Ayako Izumi, Toshihiko Ikuta, Emiko Takeoka, Sachiko Matsui, and Hitomi Mimura, Department of Neonatology, for their support in preparing the manuscript.
FUNDING INFORMATION
The authors have no specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




