SOX3 duplication in a boy with 46,XX ovotesticular disorder of sex development and his 46,XX sister with atypical genitalia: Probable germline mosaicism
Funding information: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
Abstract
Ovotesticular disorders of sex development (OT-DSD) are characterized by ovarian follicles and seminiferous tubules in the same individual, with a wide range of atypical genitalia. We report on two sibs with atypical genitalia and SRY-negative 46,XX DSD, OT-DSD was confirmed only in the boy, while the girl had bilateral ovaries. Chromosome microarray analysis (CMA) showed a 737-kb duplication at Xq27.1 including the entire SOX3 gene in both sibs, which was confirmed by quantitative real time PCR. Also, X chromosome inactivation assay showed random inactivation in both sibs. Whole exome sequencing revealed no pathogenic or likely pathogenic variant. CMA of the parents showed normal results for both, suggesting that germline mosaicism could be the reason of recurrence of this duplication in the siblings. Our results support a pathogenic role of SOX3 overexpression in 46,XX subjects leading to variable DSD phenotypes.
1 INTRODUCTION
Ovotesticular disorder of sex development (OT-DSD) is characterized by functional ovarian and testicular tissues in the same individual, either in opposite gonads or in the same gonad (ovotestis), and a wide range of ambiguous genitalia. Karyotype is mostly 46,XX, followed by mosaics or chimeras with a Y-chromosome bearing cell line, and rarely 46,XY (Syryn et al., 2021).
The estimated prevalence of 46,XX OT-DSD is 1:20,000 births or 3%–10% of all DSD (Ahmed et al., 2022). Cases of 46,XX OT-DSD often lack SRY (sex-determining region Y gene, MIM# 480000), with few cases bearing a Yp;Xp translocation including this gene. Though molecular etiology for most cases remains unknown, gain-of-function changes of pro-testicular genes or their regulatory regions or insufficient expression of pro-ovarian genes have been implicated in syndromic and nonsyndromic OT-DSD. Copy number variations (CNV) including SOX genes (SRY-related HMG-box genes) or its regulatory regions, such as SOX3 (SRY-box transcription factor 3, MIM# 313430), SOX9 (SRY-related HMG box gene 9, MIM# 608160) and SOX10 (SRY-box transcription factor 10, MIM# 602229) have also been described (Syryn et al., 2021; Vetro et al., 2015).
We report on two 46,XX sibs with ambiguous genitalia, one of them with histology-proven OT-DSD, who bear a SOX3 duplication with a strong suggestion of germline mosaicism.
2 CASE REPORTS
The first sib was a 9-month-old boy referred due to atypical genitalia. He was born at term after an uneventful pregnancy with normal birth weight (3200 g, 28th centile for a boy), length (47 cm, 12th centile) and head circumference (HC) (34 cm, 20th centile). He was the first child of healthy unrelated parents and family history was unremarkable. On physical examination, weight (9060 g, 45th centile), length (70 cm, 28th centile) and HC (44.5 cm, 29th centile) were all normal and he was non-dysmorphic. He had a 1.5-cm phallus (< −2.5 standard deviations, SD, for boys of this age [Gabrich et al., 2007]), a chordee, penoscrotal hypospadias, and both gonads were palpable in the labioscrotal folds (External Genitalia Score, EGS = 7.5 [van der Straaten et al., 2020]).
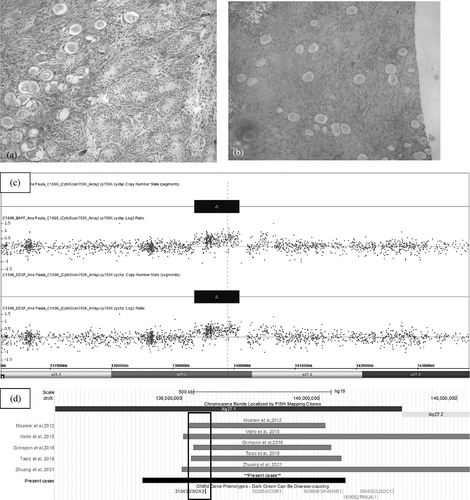
Karyotype on peripheral blood cells was 46,XX[50] and SRY gene was negative in peripheral blood leukocytes as verified by PCR. At 10 months an hCG stimulation test showed increase in testosterone concentration (from <0.2 to 1.69 ng/ml; normal response: >1.50 ng/ml). When gonadal biopsies were performed the left gonad was found to be multilobulated, but histological evaluation revealed only prepubertal testicular tissue bilaterally. When he was 19 months of age a laparoscopy revealed bilateral vas deferens and absent uterus; at the same time, the left gonad was removed due to the abnormal macroscopic appearance. Histology revealed an ovotestis (Figure 1a) and the diagnosis of OT-DSD was established.
Surgical correction of hypospadias was carried out and spontaneous puberty began at 12 years, but there were high levels of FSH (36.1 IU/L, normal range, NR, for a boy: 1.50–12.40 IU/L) and LH (11.6 IU/L, NR: 1.70–8.60 IU/L) and low testosterone (1.48 ng/ml, NR: >1.5 ng/ml) (hypergonadotropic hypogonadism). Testosterone replacement was initiated, and puberty was complete at the age of 15 years. At last clinical evaluation, at 20 years, he had a 6.5 cm-phallus, the right gonad measured 4 cm3, Tanner stage was G5P5, his height was 165 cm (9th centile) and weight 70 kg (60th centile). The height of both the father and the mother was 150 cm.
Eight years later his newborn sib was referred due to atypical genitalia. Gestation was full-term and uneventful, birth weight (2870 g, 15th centile for both boys and girls) and HC (34 cm, 29th centile) were normal, while length (44 cm) was in the 1st centile. There was a 1.8-cm phalllus (< −2.5 SD for a newborn boy), a chordee, partial labioscrotal fusion with perineal urethral opening, and gonads were not palpable (EGS = 2.5).
At 1.7 months of age (minipuberty), FSH was 8.22 IU/L (NR for a girl: 0.6–48.1 IU/L; for a boy: 0.8–5.7 IU/L) and LH 7.8 IU/L (NR for a girl: 0.1–4.7 IU/L; for a boy: 1.2–7.9 IU/L) and testosterone 1.37 ng/ml (NR for a boy: 0.84–4.78 ng/ml; for a girl: <0.71 ng/ml) (Ji et al., 2008). Karyotype on peripheral blood cells was 46,XX[50], SRY was negative, and the infant was assigned female. At 5 months bilateral gonadal biopsies revealed normal ovaries with many primordial follicles (Figure 1b). At 13 months an hCG stimulation test showed mild increase in testosterone concentration (from <0.02 to 0.52 ng/ml), suggesting the presence of testicular tissue; at that time FSH and LH before hCH were 0.90 IU/L and <0.01 IU/L, respectively. At the age of 2.5 years clitoroplasty and introitoplasty were performed together with new gonadal biopsies, which once more revealed only ovarian tissue. Thus, a 46,XX OT-DSD could not be proved.
Growth velocity was normal and there was no delay in bone age until the age of 9.5 years (height = 130.1 cm, 20th centile); from then on a decrease became apparent. At 12 years there was no spontaneous pubertal development, height was 135 cm (2nd centile), weight 44.5 kg (59th centile) and bone age 10 years. FSH was 1.44 IU/L (NR for pre-pubertal girls: 0.20–4.00 IU/L), LH < 0.10 IU/L (NR: <1.50 IU/L), estradiol <5 pg/ml (NR: <5 pg/ml), and testosterone <0.12 ng/ml (NR: <0.12 ng/ml). Hypopituitarism was ruled out based on normal levels of TSH (1.80 IU/L, NR: 0.50–4.30 IU/L), T4 (1.54 ng/dl, NR: 1.00–1.60 ng/dl), prolactin (8.17 ng/ml, NR: 4.80–23.30 ng/ml), ACTH (20.02 pg/ml, NR: 4.7–48.8 pg/ml), cortisol (9.56 μg/dl, NR: 6.20–19.40 μg/dl), and IGF1 (251.40 ng/ml, NR: 90–581 ng/ml). Magnetic resonance imaging of sella turcica also showed normal results. Estrogen replacement was initiated, and at the last visit at 13.2 years height was 145 cm (3rd centile), weight 48 kg (54th centile), bone age 11.1 years and Tanner stage B4P3.
On follow-up both sibs had normal neuromotor and speech development, no learning disabilities, and no significant health problems.
3 GENETIC ANALYSIS
Genetic tests included chromosomal microarray analysis (CMA) performed on genomic DNA from peripheral blood samples from the sibs and parents, using the CytoScan 750 K Array chip with the Reagent Kit Bundle (Affymetrix). SOX3 copy number variation was also estimated using relative quantification Real-time PCR (qPCR) analysis (Hs00551858_cn, ThermoFisher Scientific). SNP genotyping data from sibs and both parents, using the ChAS software (Affymetrix), was applied to confirm paternity and maternity, according to the ChAS User Guide (Available at https://www.thermofisher.com/br/en/home/life-science/microarray-analysis/microarray-analysis-instruments-software-services/microarray-analysis-software/chromosome-analysis-suite. Accessed on January 17, 2020). X chromosome inactivation assay was performed on DNA extracted from blood cells of the sibs by methylation sensitivity analysis of highly polymorphic (CAG)n region of the human androgen receptor gene (AR) in Xq12, according to a standard protocol (Jones, 2014).
DNA library preparation, sequencing and data generation for exome sequencing were performed by Novogene Bioinformatics Technology Co. DNA libraries were prepared using an Agilent SureSelect Human All Exon V6 kit (Agilent Technologies). One hundred and fifty-paired-end sequencing was performed on a HiSeq 2500 system (Illumina), with a mean coverage of 100×. The following filters were applied for variant calling: (1) variants with minor allele frequencies above 1% in the main population data banks were excluded; (2) synonymous variants and intronic variants not located within splice site regions were excluded; (3) the variants were first analyzed in 106 genes known to be related to gonadal or genital development, or related to steroidogenesis, including target genes recently associated with DDS-OT, including NR5A1, NF2R2, WT1, SOX genes, WNT4 and RSPO1. A comprehensive description of methods is depicted as Appendix S1.
4 RESULTS
CMA showed a duplication of 737 kb on Xq27.1 in both sibs—(arr[GRCh37]Xq27.1(139357310_140094746)x3). The duplication includes the entire SOX3 gene and two other OMIM genes with no associated phenotype (CDR1-AS and CDR1) (Figure 1c). Neither parent carried this duplication. Paternity and maternity were confirmed by SNP genotyping data. The Mendelian error checking tool from ChAS software (Affymetrix) showed a Role Validity of 1 for every sample pair analyzed (mother/sib one, father/sib one, mother/sib two, and father/sib two), which confirms that all of them are related. In addition, after trio analysis for each sib, the number of SNPs with Mendelian error was only 1676/200,484 (0.84%) for sib one and 740/200,484 (0.37%) for sib two, also confirming paternity and maternity. This low percentage of Mendelian error is probably occurred due to genotyping errors during hybridization procedures, and is acceptable for any SNP. Quantitative PCR for copy-number variation confirmed the duplication in both sibs, and X inactivation was random for both.
After a rigorous analysis of target genes and classification of the variants according to the American College of Medical Genetics and Genomics (ACMG) Standards and Guidelines (Richards et al., 2015), WES revealed no pathogenic or likely pathogenic variants.
5 DISCUSSION
Differentiation of the primitive gonad into testis or ovary depends on genetic sex. Subsequently, in the presence or absence of testicular hormones, internal and external genitalia will follow the male or female pathway. Gonadal determination relies not only on the presence or absence of SRY, but also on a complex gene network, with either activation of the testis pathway and simultaneously repression of the ovarian pathway or vice versa (Rey et al., 2020). Therefore, pathogenic variants in several genes have been associated to disorders of gonadal differentiation in both XY and XX subjects.
The main role of SRY is to upregulate SOX9, which triggers Sertoli cell differentiation. Overexpression of SOX9 and other SOX genes (SOX10, SOX3) due to CNV harboring these genes or their regulatory regions, and genomic rearrangements altering expression due to position effects have been shown to induce testicular differentiation in SRY-negative XX subjects (Croft et al., 2018; Grinspon & Rey, 2016; Vetro et al., 2015).
SOX3 has a single exon and maps to a highly conserved region of X chromosome (Xq27.1). It is expressed in brain, pituitary and gonads and encodes a protein very similar to SRY (Sutton et al., 2011). Duplications at Xq27.1 harboring SOX3 in 46,XY subjects have been associated with X-linked hypopituitarism, including growth hormone, gonadotropins and other hormone deficiencies, intellectual disability, and neural tube defects (Arya et al., 2019). Most cases are maternally inherited. However, the mothers usually exhibit a normal phenotype, which may be due to skewed inactivation of the affected X chromosome (Arya et al., 2019; Hol et al., 2000); low penetrance of DSD caused by SOX3 overdosage has also been suggested (Igarashi et al., 2015).
The first descriptions of XX sex reversal due to SOX3 duplications, together with a study with transgenic mice model, suggested that in XX subjects SOX3 can act as SRY via increased dosage or ectopic expression. It was shown that SOX3 can act synergistically with SF1 to upregulate SOX9 expression and activate the testicular differentiation cascade (Sutton et al., 2011). Since then, a few more cases of SOX3 duplication in 46,XX subjects leading to OT-DSD or testicular DSD have been described.
XX testicular DSD is characterized by bilateral testes, usually normal male phenotype, microorchidism and azoospermia, and 10% of cases are SRY-negative (Grinspon & Rey, 2016). Patients with testicular DSD due to SOX3 duplication were referred due to infertility, atypical genitalia, development delay or kidney anomaly. Two of them were de novo, and in the remaining, inheritance was unknown (Moalem et al., 2012; Sutton et al., 2011; Tasic et al., 2019; Vetro et al., 2015) (Table 1).
| Reference | Present cases | Sutton et al. (2011) | Moalem et al. (2012) | Vetro et al. (2015) | Grinspon et al. (2016) | Tasic et al. (2019) | Zhuang et al. (2021) | ||
|---|---|---|---|---|---|---|---|---|---|
| Index | Sister | Patient A | Patient C | ||||||
| Clinical picture | |||||||||
| Age at 1st visit | 9 mo | 1 mo | 30 y | 19 mo | 5 mo | 8 y | 2.5 y | 11 y | 7 y |
| Referral reason | AG | AG | Infertility | Failure to thrive, DD | AG | DD | AG | Hypoplastic R kidney + CHyp | AG |
| Sex assignment | Male | Female | Male | Male | Male | Male | Male | Male | Male |
| Genitals | Phallus: 1.5 cm (9 mo); 6.5 cm (20 y); PSHyp | Phallus: 1.8 cm (1 mo); SHyp; partial LS fusion | NR | Phallus: 3.4 cm (19 mo), hypoplastic scrotum | PSHyp, underdeveloped bifid scrotum | Normal male genitalia | Phallus: 3.2 cm (2.5 y); CHyp | Phallus: 5 cm (11 y), CHyp | CHyp |
| Gonads (location, type, volumea) | R: LS, T, 4 cm3 (20 y); L: LS, OT | BL: NP, O | T volume ~ 5 cm3b | BL: retractile Tb | R: LS, T; L: IR,Tb | NR | BL: NP, OT | T volume >4 cm3b | R: NP, T; L: NP, OT |
| Uterus | (−) | (+) | NR | NR | NR | NR | L: rudimentary Müllerian derivatives | Absent | NR |
| Puberty | Induced, hypergonadotropic hypogonadism | Induced, hypogonadotropic hypogonadism | Normal secondary sexual characteristics | N/A | N/A | N/A | N/A | NR | N/A |
| Other health problems | (−) | Asthma | Microcephaly, Growth retardation | (−) | Sleep disturbance | (−) | (−) | (−) | |
| Diagnosis | OT-DSD | XX DSD | Testicular DSD | Testicular DSD | Testicular DSD | Testicular DSD | OT-DSD | Testicular DSD | OT-DSD |
| SOX3 duplication | |||||||||
| Chromosome region | Xq27.1 | Xq27.1 | Xq27 | Xq27.1 | Xq27.1q27.3 | Xq27.1 | Xq27 | Xq27.1q27.2 | |
| Genome reference | GRCh37/hg19 | NR | NR | GRCh36/hg18 | GRCh37/hg19 | GRCh37/hg19 | GRCh36/hg18 | GRCh37/hg19 | |
| Genomic positions | 139,357,310_140,094,746 | NR | NR | 139,354,859_139,848,664 | 139,504,721_145,120,304 | 139,541,737_140,043,863 | 139,360,520_139,908,320 | 139,499,778_141,777,782 | |
| Duplication size | 737 kb | 123 kb | 6 Mb | 494 kb | 5.6 Mb | 0.5 Mb | 550 kb | 2.2 Mb | |
| Genes | SOX3, CDR1-AS, CDR1 | SOX3 | SOX3 + ≥ 18 additional distally located genes | SOX3,RP1-177G6.2,CDR1, MIR320D2 | SOX3 + other genes (not specified) | SOX3, RPS17P17, CDR1, MIR320D2 | SOX3, RP1-177G6, CDR1 | CDR1, LDOC1, MAGEC1, MAGEC2, MAGEC3, SOX3, SPANXA1, PANXA2, SPANXB1 SPANXB2, SPANXC, SPANXD | |
| Inheritance | Inherited (germline mosaicism) | Unknown | Unknown | De novo | De novo | De novo | Unknown | De novo | |
- Abbreviations: AG = atypical genitalia; BL = bilateral; CHyp = coronal hypospadias; DD = developmental delay; DSD = disorders of sex development; IR = inguinal region; L = left; LS = labioscrotal; mo = months; N/A = not applicable; NP = nonpalpable; NR = not reported; OT = ovotestis; PSHyp = penoscrotal hypospadias; R = right; SHyp = scrotal hypospadias; T = testis; y = years.
- a When available in case of testis.
- b No information on histologic studies.
Two patients with SOX3 duplication and OT-DSD had been already described; they had atypical genitalia and de novo CNV. There was no reference to short stature, developmental delay or dysmorphic picture in these cases, as well as in the present cases (Grinspon et al., 2016; Zhuang et al., 2021) (Figure 1d, Table 1).
In the present family OT-DSD could not be confirmed in the sister, though the increase in testosterone concentration after hCG stimulation indicated the presence of testicular tissue. There were no further attempts to look for testicular tissue and remove it in order to avoid the risk of removing a significant part of normal ovarian tissue and compromise future ovarian function. Instead, we chose to have a close follow-up to detect signs of virilization at puberty, which did not happen so far.
Both sibs had atypical genitalia, but gonadal histology and function were strikingly different. While the boy had predominance of testicular tissue, absent uterus (indicating normal embryonic Sertoli cell function) and hypergonadotropic hypogonadism, his sister's biopsies revealed only normal ovarian tissue, she had a normal uterus and pubertal delay. Although both sibs showed a random pattern of X chromosome inactivation, we cannot rule out an influence of skewed X inactivation on the phenotypic differences, since X inactivation patterns may be distinct among different tissues (Cotton et al., 2015). It is worth noting that SOX3 duplication led to pituitary hormone deficiencies in 46,XY subjects, and one may hypothesize that pubertal delay in the sister may be due to gonadotrophin deficiency, which, in turn, could be due to skewed X inactivation in the pituitary.
Since CMA of the parents revealed normal results for both and SNP genotyping analysis allowed us to rule out non-paternity, we hypothesize that germline mosaicism could be the reason of recurrence of this duplication in the siblings. This unique case of probable germline mosaicism of a SOX3 duplication provides further evidence for a pathogenic role of SOX3 overexpression in SRY-negative 46,XX subjects, with different DSD phenotypes. It also reinforces the importance of CMA in routine investigation of patients with disorders of gonadal development.
AUTHOR CONTRIBUTIONS
Experiment conduction: Flávia Marcorin de Oliveira, Beatriz Amstalden Barros, Ana Paula dos Santos, Nilma Lúcia Viguetti Campos, Taís Nitsch Mazzola, Paulo Latuf Filho. Data analysis: Flávia Marcorin de Oliveira, Beatriz Amstalden Barros, Ana Paula dos Santos, Taís Nitsch Mazzola, Liliana Aparecida Lucci De Angelo Andrade, Mara Sanches Guaragna, Maricilda Palandi de Mello, Társis Antonio Paiva Vieira. Study design: Mara Sanches Guaragna, Maricilda Palandi de Mello, Társis Antonio Paiva Vieira, Gil Guerra-Junior, Andréa Trevas Maciel-Guerra. Data collection: Flávia Marcorin de Oliveira, Beatriz Amstalden Barros, Taís Nitsch Mazzola, Liliana Aparecida Lucci De Angelo Andrade, Mara Sanches Guaragna, Maricilda Palandi de Mello, Társis Antonio Paiva Vieira, Gil Guerra-Junior, Andréa Trevas Maciel-Guerra. Manuscript preparation: Flávia Marcorin de Oliveira, Társis Antonio Paiva Vieira, Gil Guerra-Junior, Andréa Trevas Maciel-Guerra. Providing patient care and collating the clinical data: Beatriz Amstalden Barros, Gil Guerra-Junior, Andréa Trevas Maciel-Guerra.
ACKNOWLEDGMENTS
This work was supported by CAPES (Finance Code 001) and FAPESP (grant 2019/26382-3).
CONFLICT OF INTEREST
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article




