A diagnosis of Birt–Hogg–Dubé syndrome in individuals with Smith–Magenis syndrome: Recommendation for cancer screening
Funding information: Intramural Research Program of the Center for Cancer Research, National Cancer Institute; Intramural Research Program of the National Human Genome Research Institute; Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases; National Institutes of Health; Brown University
Abstract
We report a series of four unrelated adults with Smith–Magenis syndrome (SMS) and concomitant features of Birt–Hogg–Dubé (BHD) syndrome based upon haploinsufficiency for FLCN and characteristic renal cell carcinomas and/or evidence of cutaneous fibrofolliculomas. Three of the cases constitute the first known association of histopathologically verified characteristic BHD-associated renal tumors in adults with SMS; the fourth was identified to have histologically confirmed skin fibrofolliculomas. Molecular analysis documented second-hit FLCN mutations in two of the three cases with confirmed BHD renal pathology. These cases suggest the need to expand management recommendations for SMS to include kidney cancer surveillance starting at 20 years of age, as per the screening recommendations for BHD syndrome.
1 INTRODUCTION
Birt–Hogg–Dubé (BHD) syndrome (MIM 135150) is a rare autosomal dominant genodermatosis characterized by increased risk of cutaneous fibrofolliculomas (FF), pulmonary cysts, spontaneous pneumothorax, and renal tumors. Manifestations of BHD typically do not develop until adulthood, with variability in age of onset. Fibrofolliculomas or trichodiscomas typically develop on the face, neck, and upper body after age 25; they are an important clinical clue to a diagnosis of BHD, typically becoming more prominent in the fourth and fifth decades of life. Pulmonary cysts, which are identified in 89% of individuals with BHD, generally occur after age 30 years (median age 38 years; range 22–71 years) (Toro et al., 2008) and may go unrecognized; however, these are associated with an increased risk for a more serious condition, spontaneous pneumothorax, which is documented in about 30% of adults with BHD usually before age 40 years (Schmidt & Linehan, 2015). Renal tumors are the most life-threatening manifestation of BHD syndrome and are seen in 14%–34% of individuals with BHD between the ages of 20–74 years, with a mean age of renal cell carcinoma (RCC) diagnosis of 50.3 years (Barrisford et al., 2011; Menko et al., 2009, Pavlovich et al., 2005; Shuch et al., 2014). Renal tumors in BHD syndrome typically have characteristic hybrid oncocytic (50%) or chromophobe (34%) pathology (Pavlovich et al., 2002). However, clear cell RCC (9%) or oncocytoma (5%) also may occur (Barrisford et al., 2011; Pavlovich et al., 2002). Consistent with hereditary predisposition, the cancer is multifocal or bilateral more than half of the time (Pavlovich et al., 2005). Surveillance via abdominal imaging, beginning at age 20 and continuing every 3 years, has been recommended (Menko et al., 2009; Stamatakis et al., 2013). MRI has been recommended for abdominal screening in patients affected by or at risk for BHD in order to limit the long-term burden of radiation, while ultrasonography is not recommended as the tumors may have similar echogenicity to the surrounding renal parenchyma (Stamatakis et al., 2013). The recommendation is to monitor the tumors until the largest tumor approaches the 3 cm threshold, at which time surgical resection may be recommended (Herring et al., 2001; Metwalli & Linehan, 2014).
In 2001, genetic linkage analysis demonstrated that the chromosomal 17p11.2 region contained the BHD-associated gene (Schmidt et al., 2001). The following year, germline pathogenic variations in the FLCN gene were identified in individuals affected with the clinical manifestations of BHD, confirming that FLCN haploinsufficiency poses a risk to develop BHD (Nickerson et al., 2002). Folliculin, the product of the FLCN gene, is a tumor suppressor involved in varied metabolic pathways and cellular processes, including modulation of the mTOR pathway, regulation of PGC1α and mitochondrial biogenesis, and negative regulation of TFE3/TFEB transcriptional activity (Schmidt and Linehan, 2018). Germline FLCN pathogenic variations account for 88% of BHD cases, with the majority due to frameshift (deletion/insertion), nonsense, or splice site mutations (Toro et al., 2008, Schmidt and Linehan, 2018). However, large intragenic deletions or duplications of FLCN in the germline of individuals affected by BHD have also been described (Benhammou et al., 2011; Schmidt & Linehan, 2015).
Smith–Magenis syndrome (SMS) was first associated with a microdeletion of 17p11.2 (Smith et al., 1986) that accounts for 90% of diagnosed cases, with the majority reflecting a common 3.7 Mb deletion. Haploinsufficiency for RAI1 by heterozygous deletion or point mutation identified RAI1 as the gene responsible for the majority of the SMS phenotype (Elsea & Girirajan, 2008; Gropman et al., 2007). RAI1 is a dosage-dependent transcription factor (Carmona-Mora et al., 2012). SMS is observed in the general population at a rate of 1/15,000–1/25,000; it is typically de novo and is clinically characterized by a distinctive facial appearance, varying degrees of developmental delay and cognitive impairment, as well as a distinct behavioral phenotype, including sleep disturbance, stereotypic behaviors, hyperactivity, aggressive and self-injurious behaviors, and hyperphagia (problems regulating food intake) (Elsea & Girirajan, 2008, Edelman et al., 2007, Gropman et al., 2007; Smith et al., 1998). Microarray is the best initial diagnostic test; however, if the deletion is not seen and there remains a strong clinical suspicion, consideration should be given to further molecular genetic testing, including single gene sequencing or multigene panel that includes RAI1, or whole exome or genome sequencing.
Although 17p11.2 is an unstable region within which rearrangements are frequently seen in sporadic cases of cancer, individuals with SMS have not been considered to have a greater cancer risk than the general population. Cancer has only occasionally been reported in SMS and is not considered part of the typical phenotype (Elsea & Girirajan, 2008; Hienonen et al., 2005; Scheurlen et al., 1997). The FLCN gene maps within the common SMS del 17p11.2 region. As the majority of SMS cases result from a common deletion interval spanning 3.7 Mb in 17p11.2 that includes the FLCN gene (Elsea & Girirajan, 2008), there has been a theoretical concern for increased kidney cancer risk in SMS patients; however, individuals with SMS with the common deletion have not routinely undergone renal screening as recommended for individuals with germline FLCN variations and a diagnosis of BHD syndrome.
Reports of the co-occurrence of features of BHD in individuals with SMS are limited to a few case reports. In 2016, Dardour et al. described a 57-year-old man with SMS due to a 3.47 Mb deletion incidentally found to have bilateral renal tumors; a renal biopsy was not performed, and skin and lung findings of BHD were absent (Dardour et al., 2016). Spontaneous pneumothorax was first reported by Truong et al. (2010), who identified an 18-year-old male with SMS due to the RAI1 mutation; he had no other features of BHD aside from three episodes of pneumothorax between ages 5–10 years. The prevalence of BHD symptoms in SMS was explored in a recent survey of families affiliated with the international advocacy support organization PRISMS (Parent and Researchers Interested in SMS) (Finucane et al., 2021). This study relied on online patient-entered data without confirmatory medical record review and queried the three hallmark organ systems involved in BHD syndrome (i.e., spontaneous pneumothorax; pathognomonic skin features including fibrofolliculoma, trichodiscoma, and/or skin tags; and renal cancer). Among 117 respondents less than 20% of patients were 30 years of age or older (median age: 22.42 years; range: 6 months–49 years). Seven (5.98%) reported at least one of the three hallmark features of BHD. Specifically, spontaneous pneumothorax (four of five occurred before age 10 years) was reported for five SMS cases, and skin lesions specifically identified as fibrofolliculoma were reported for two SMS cases (age 20 and 21 years, respectively); however, no cases of renal cancer were reported (Finucane et al., 2021). Thus, the actual risk of renal cell cancer in individuals with SMS is still unknown.
We report the co-occurrence of BHD syndrome in SMS adults, including two previously described abstract cases (patients 1 and 2; Smith et al., 2014) and two additional adult females; all four presented with symptoms of BHD in adulthood. Three presented with renal masses consistent with BHD after age 40 years, while the youngest, a 25-year-old female, presented with biopsy proven facial fibrofolliculomas, a suspected lung cyst, and a normal renal MRI scan.
2 PARTICIPANTS AND METHODS
2.1 Participants
This study utilizes data collected from patients enrolled in a longitudinal observational study at the National Institutes of Health under IRB-approved protocol 01-HG-0109 “Natural History Study of the Clinical and Molecular Manifestations of Smith–Magenis syndrome (SMS)”. Informed consent was obtained prior to enrollment from the parent/guardian.
2.2 Clinical, molecular, and pathologic data
Medical records, including outside imaging studies, surgical notes, and pathology reports and slides, were reviewed by the NIH SMS Research Team with consultative input from the National Cancer Institute/Urologic Oncology Branch at the NIH with expertise in RCC and BHD. Pathology review and molecular analysis of slides from patients 1, 2, and 4 were performed at the NCI by MJM. Sanger sequencing was conducted at the CCR Genomics Core at the National Cancer Institute, NIH. Deletion size was determined by molecular SNP-chip analysis performed on genomic DNA samples by the NHGRI Genomics Core, NIH.
3 RESULTS
The clinical, molecular, and pathologic findings of four SMS adults (3F/1M) are summarized in Table 1. All four patients have documented deletions of 17p11.2 that encompass both the RAI1 and FLCN genes, resulting in haploinsufficiency. Patients 1 and 2 were previously reported at the 64th annual meeting of the American Society of Human Genetics in 2014 (Smith et al., 2014, ASHG).
PATIENT 1.
A female with a complex medical history presented to a genetics clinic at age 50 11/12 years and was cytogenetically diagnosed with SMS (del 17) (p11.2p11.2) (Table 1). The physical exam was notable for typical phenotypic and behavioral features of SMS. She was referred for gynecologic evaluation due to a suspected uterine anomaly; pelvic MRI revealed a left kidney mass; abdominal computed tomography (CT) showed involvement of both the left and right kidneys; and she subsequently underwent left nephrectomy and partial right kidney resection. Her exam was negative for BHD-associated skin lesions; previous chest x-rays had not shown lung cysts. Chest CT was not performed.
| Case 1a | Case 2a | Case 3 | Case 4b | Dardour et al., 2016 | |
|---|---|---|---|---|---|
| Gender | Female | Male | Female | Female | Male |
| Age at SMS diagnosis | 50y | 37y | 7y | 16y | 49y |
Deletion size (breakpointsc) |
3.67 Mb (16.6–20.3) |
7.09 Mb (14.4–21.5) |
6.32 Mb (15.17–21.5) |
3.77 Mb (16.5–20.3) |
3.47 Mb (16.7–20.2) |
| Age at last evaluation | 50y |
45y |
25y |
41y |
57y |
Anthropometry Weight (kg) Height (cm) BMI (kg/M2) |
76 kg 151 cm 33.3 |
65.8 kg 167 cm 24.4 |
69.75 kg 160.2 cm 27.2 |
75.7 kg; 147.3 cm 34.9 |
(49y) 57 kg 155 cm 23.7 |
| Renal pathology | Bilateral hybrid oncocytic tumors | R oncocytosis L oncocytic neoplasm |
Normal MRI at 24y |
R oncocytoma (3.6 cm); confirmed chromophobe |
Multiple bilateral renal tumors (not biopsied) |
“Second-hit” FLCN mutation |
Path slides no longer available | FLCN point mutation (c.736delA; S246fs) |
NA | FLCN splice site variant (c.1432 + 1 G > C) likely pathogenic | Not studied |
| Cutaneous findings | None noted | None noted on autopsy | Biopsy confirmed FF | Extreme eczema from “drooling”; no facial skin lesions noted | Psoriasis; no skin tumors |
| Lung findings | None noted | None noted on autopsy | 4 mm cyst | None noted | None noted |
- Abbreviations: NA, not applicable; FF, fibrofolliculoma; y, years.
- a Cases previously described by Smith et al., 2014 (ASHG abstract).
- b Case 4: TruSight Oncology Gene Panel.
- c Constitutional/germline deletion breakpoints (build hg19).
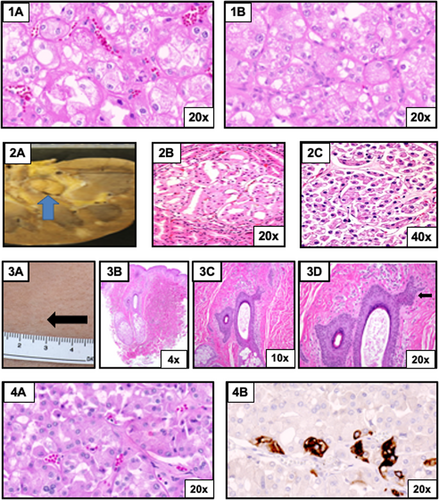
Tumor slides were sent to the NIH for pathology review, documenting bilateral hybrid oncocytic tumors (Figure 1 [1A–B], top row), consistent with BHD (Smith et al., 2014, ASHG). Comparative genome hybridization (CGH) microarray analysis of blood DNA confirmed a 3.67 Mb deletion (chr17:16,660,721-20,331,131; build hg19). The BHD diagnosis is based on haploinsufficiency of FLCN, coupled with characteristic kidney pathology; pathology slides are no longer available to permit molecular reanalysis of the tumor tissue to assess for a pathogenic variant in the remaining somatic copy (“second-hit”) of the FLCN gene.
PATIENT 2.
Patient 2 (Table 1) is a male initially diagnosed with SMS at the of age 37 years. His germline 7.1 Mb deletion was documented to be secondary to an inherited deletion from his mosaic mother (Smith et al., 2006, ASHG). At the age of 45 years, he presented to the emergency room complaining of right upper quadrant pain and bilateral leg swelling. He was found to have anemia and occult blood in his stool. A CT scan with contrast showed diffuse gastric wall thickening and anasarca. Subsequent endoscopy showed a proximal gastric mass. The biopsy revealed a high-grade B-cell lymphoma with medium to large cells, a high-mitotic index, and numerous apoptotic cells. The infiltrate focally destroyed the background gastric mucosa. Rearrangements of c-myc and Bcl-6 were detected by fluorescence in situ hybridization (FISH). Morphology, immunophenotype, and cytogenetics were consistent with unclassifiable B-cell lymphoma, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Positron emission tomography showed diffuse 18F-fluorodeoxyglucose (FDG)-avid disease above and below the diaphragm. Chemotherapy was declined, and the patient died 1 month after diagnosis. A full autopsy revealed diffuse B-cell lymphoma involvement of abdominal and thoracic viscera including liver, spleen, mesenteric and retroperitoneal lymphoid tissue, gastric wall, diaphragm, appendix, pericardium, and omentum. A 1 cm renal lesion was identified in the left kidney, which was described in the autopsy report as an oncocytoma; multiple cortical and medullary nodules of up to 0.7 cm, reported as being most consistent with lymphoid infiltrates, were also seen in both kidneys. No mention of lung cysts or abnormal skin findings was noted on autopsy. Slides of the renal and liver specimens were sent to the NIH for molecular testing and pathology review (Figure 1 [2A–C], second row). Pathology review of slides from the kidney at the NIH revealed an oncocytic neoplasm with features of chromophobe RCC; evidence of malignant lymphoma was also identified (Smith et al., 2014). CGH microarray analysis of blood DNA documented a large germline 7.1 Mb deletion (chr17:14,381,188-21,470,731; build hg19), with a second, somatic FLCN mutation (NM_144997.7: c.736delA; p.Ser246Valfs*17) identified in the renal specimen (Figure S1), but not in the liver specimen, demonstrating that this frameshift mutation was a renal tumor-associated somatic second hit.
PATIENT 3.
Patient 3 is a 25-year-old female with cognitive dysfunction and speech delay, followed longitudinally at NIH. She was found to have the phenotypic and behavioral features characteristic of SMS. At age 25 years, outside studies identified a possible lung cyst and cutaneous lesions suggestive of fibrofolliculoma, prompting re-evaluation (Table 1). Germline CGH microarray analysis of blood DNA identified a 6.3 Mb 17p11.2 deletion (Chr17:15,175,570-21,503,147; build hg19). Abdominal MRI (with and without contrast) did not show evidence of renal masses. NIH review of outside lung CT films obtained at age 23 years revealed minimal right pleural effusion and lower lung atelectasis and/or scarring. Follow-up chest CT w/o contrast at 25 years noted a new nonspecific 4 mm cyst within right lung base and unchanged small right pleural effusion unchanged without evidence of pneumothorax. Cutaneous examination showed open comedones on the lateral aspects of the cheeks and multiple small white papules on the lateral face and left neck area, clinically suspicious for fibrofolliculoma (Figure 1 [3A], third row). Skin biopsy of a left neck papule revealed epithelial strands emanating from a hair follicle in the dermis consistent, with fibrofolliculoma (Figure 1 [3B–D], third row). The remainder of her dermatologic exam was noncontributory. Routine lab studies were normal, with the exception of mildly elevated alanine aminotransferase.
PATIENT 4.
Patient 4 is a 42-year-old adult female with a history of developmental delay and cognitive dysfunction, and SMS diagnosis was confirmed cytogenetically by FISH at age 16 (Table 1). NIH evaluation at age 27 documented typical phenotypic and neurobehavioral features of SMS; DNA-based microarray confirmed a common 3.77 Mb deletion (chr17:16,516,749-20,292,395; build hg19). At age 41, she was incidentally diagnosed with a right renal mass (asymptomatic) on a CT performed for the evaluation of abdominal bruising from “accidental injury”. Further CT imaging with contrast confirmed a solid right renal mass (3.6 cm) located in the mid pole without evidence of multifocal disease or disease in the contralateral kidney. Chest CT (w/o contrast) identified irregular opacities in the right middle and upper lobes, in addition to patchy ground glass opacities in the right lung. Morphology was suggestive of a chronic infectious or inflammatory process; however, on a repeat chest CT 10 months later, the opacities were no longer present, indicating resolved inflammation. Neither CT revealed cystic lesions. She underwent a right robotic-assisted partial nephrectomy at an outside institution. The renal mass tissue was reported as oncocytoma by an outside pathologist. CK7 immunostaining showed rare patchy expression. Pathology slides obtained for review and immunostaining by NIH favored the diagnosis of chromophobe RCC based on patchy CK7 expression and negative c-KIT staining (Figure 1 [4A–B], fourth row); TruSight Oncology 500 Gene Panel subsequently identified a likely pathogenic FLCN splice site variant (c.1432 + 1G > C) (Figure S1); variant allele frequency 74% (210/284 reads) in the tumor (estimated tumor content: 80%).
4 DISCUSSION
To our knowledge, this series constitutes the first report of concurrent BHD syndrome in SMS adults diagnosed based on a combination of characteristic tumor pathology and germline deletion of FLCN, with molecular analysis identifying a second-hit FLCN mutation in two of three cases with RCC. One of the four patients was documented to have a cutaneous lesion that was biopsy confirmed to be fibrofolliculoma at age 25 years, but this patient showed no renal abnormalities on imaging.
According to one proposed diagnostic criterion for BHD syndrome, haploinsufficiency of FLCN via the typical deletion seen in SMS (or a pathogenic FLCN mutation) is sufficient for a diagnosis of BHD syndrome (Menko et al., 2009); however, other clinicians reserve the diagnosis of BHD syndrome to FLCN haploinsufficiency in the presence of one or more characteristic phenotypic manifestations. There has been a theoretical concern for increased renal cancer risk in patients with SMS since the majority of SMS cases result from a 3.7 Mb deletion in 17p11.2, which includes the FLCN gene; this concern was heightened by the report of a 57-year-old man with SMS and bilateral renal tumors identified incidentally during a CT scan for hip pain. While pathology was not obtained from the tumors, he was presumed to have BHD based on his preexisting deletion and the presence of renal tumors (Dardour et al., 2016). However, to our knowledge, there have not been any previously reported cases of pathologically confirmed BHD-characteristic tumors in SMS patients. Patient 1 in this study had bilateral hybrid oncocytic tumors, which are commonly seen in BHD patients but are not generally found outside of this syndrome. The chromophobe RCC described in patients 2 and 4 is a tumor type seen in about 33% of BHD renal tumors, compared with about 5% of sporadic RCCs (Davis et al., 2014). The identification of second-hit FLCN mutations in renal tumors from patients 2 and 4 is strong corroboration for the diagnosis of BHD syndrome. MRI of the youngest adult (patient 3) showed no evidence of renal abnormalities/tumors at age 25; however, the presence of the typical cutaneous fibrofolliculomas (FF), confirmed by biopsy, is consistent with the BHD diagnosis and supports the need for ongoing surveillance in this patient for potential renal tumors in the future.
Our clinical observations suggest the need for kidney cancer surveillance in individuals with SMS secondary to deletions that include FLCN. It is notable that in two adult SMS patients over 40 years, asymptomatic renal tumors were identified by imaging performed for other reasons (Table 1: Case 4 and Dardour et al., 2016). To assure timely diagnosis and evaluation of renal tumors in SMS adults carrying a deletion in FLCN, we recommended surveillance by baseline abdominal MRI or CT scan with/without contrast, beginning at age 20 years and continuing every 3 years thereafter (Menko et al., 2009; Stamatakis et al., 2013). Long-term surveillance using MRI is recommended to minimize the amount of radiation exposure. When a renal mass is detected, imaging intervals will depend on the location, size, and growth rate of the tumors (Schmidt & Linehan, 2015).
It is estimated that >80% of individuals with BHD syndrome develop cutaneous findings, 83%–85% develop pulmonary cysts, 28%–38% have at least one spontaneous pneumothorax, and 14%–35% develop RCC, which has been observed in patients as young as age 20 years (Barrisford et al., 2011, Schmidt et al., 2005, Toro et al., 2008). As the majority of cases of SMS are secondary to a deletion that encompasses FLCN as well as RAI1, this raises the possibility that the pulmonary, skin, and renal manifestations of BHD have been underappreciated in individuals with SMS or that BHD manifestations are of relatively low penetrance in SMS. Additionally, most early studies of SMS included infant to adolescent ages with fewer older adults within the age range in which BHD-associated renal and cutaneous tumors develop. In order to distinguish between these possibilities, further study of the natural history into adulthood is needed to specifically evaluate for manifestations of BHD syndrome in individuals with SMS.
The recent exploratory online parent response survey conducted by Finucane et al. (2021) assessed the frequency of three hallmark features of BHD (i.e, spontaneous pneumothorax, renal cancer and/or pathognomonic skin lesions) among 117 individuals with SMS (median age 22.4 years; range 6 months to 49 years). Symptoms of BHD were reported for seven individuals with SMS, including spontaneous pneumothorax in five (four occurred before age 10 years) and skin lesions in two in their early 20s; however, no documented cases of renal cancer were reported. Although limited by the lack of confirmatory medical record review and by individuals whose age falls below the mean age of onset of renal tumors in BHD (50.3 years), this study lends support to the need for further diagnostic consideration in SMS, with implications for medical surveillance for individuals with SMS into adulthood.
AUTHOR CONTRIBUTIONS
All authors have significantly contributed to the work, providing written content toward the first draft of the article according to their subject expertise. All authors read and approved the final version as submitted and agree to be accountable for all aspects of the work. Cathy D. Vocke: Design and conceptualization of the study; acquisition, molecular genetic analysis, and interpretation of the data, including tables and figures; drafting and critical review of the manuscript. Leah R. Fleming: Design and conceptualization of the study; acquisition, analysis, and interpretation of the clinical data; and drafting and revising the manuscript. Anna M. Piskorski: Acquisition, analysis, and interpretation of the histopathological data. Ali Amin: Acquisition, analysis, and interpretation of the histopathological data; revising the manuscript. Chanika Phornphutkul: Acquisition, analysis, and interpretation of the clinical data; revising the manuscript. Suzanne de la Monte: Acquisition, analysis, and interpretation of the clinical and pathological data; revising the manuscript. Thierry Vilboux: Acquisition, analysis, and interpretation of molecular genetic data; revising the manuscript. Folami Duncan: Acquisition of clinical data from medical records and conducting an initial literature search; revising the manuscript. Joan Pellegrino: Acquisition, analysis, and interpretation of the clinical data; revising the manuscript. Bonnie Braddock: Acquisition, analysis, and interpretation of the clinical data; revising the manuscript. Lindsay A. Middelton: Acquisition, analysis, and interpretation of the data; drafting and revising the manuscript. Laura S. Schmidt: Acquisition, analysis, and interpretation of the data, including tables and figures; drafting and critical review of the manuscript. Maria J. Merino: Acquisition, analysis, and interpretation of the histopathological data; drafting and revising the manuscript. Edward W. Cowen: Acquisition, analysis, and interpretation of the data, including tables and figures; drafting and revising the manuscript. Wendy J. Introne: Design and conceptualization of the study; acquisition, analysis, and interpretation of the data, including tables and figures; drafting and revising the manuscript. W. Marston Linehan: Acquisition, analysis, and interpretation of data, including tables and figures; revising and critical review of the manuscript. Ann C. M. Smith: Design and conceptualization of the study; acquisition, analysis, and interpretation of the data, including tables and figures; drafting, critical review, and finalizing the manuscript with the inclusion of all revisions for submission.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Human Genome Research Institute, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. There are no conflicts of interest to declare. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors wish to acknowledge the laboratory support of Settara Chandrasekharappa, PhD, and Frank Donovan, PhD, Genomics Core of the National Human Genome Research Institute; the CCR Genomics Core at the National Cancer Institute; Dr. Chyi-Chia Richard Lee, MD, PhD, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute; and post humous recognition of early contributions and pathological findings from Dr. Ed Stopa, Department of Pathology, Warren Alpert Medical School of Brown University. The authors also thank the patients with Smith–Magenis syndrome and their families.
CONFLICT OF INTEREST
None of the authors have a conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




