Growth charts for Marfan syndrome in the Netherlands and analysis of genotype–phenotype relationships
Funding information: Contactgroep Marfan Nederland; Emma Children's Hospital Foundation (WAR2017-24)
Abstract
To optimize care for children with Marfan syndrome (MFS) in the Netherlands, Dutch MFS growth charts were constructed. Additionally, we aimed to investigate the effect of FBN1 variant type (haploinsufficiency [HI]/dominant negative [DN]) on growth, and compare MFS-related height increase across populations. Height and weight data of individuals with MFS aged 0–21 years were retrospectively collected. Generalized Additive Models for Location, Scale and Shape (GAMLSS) was used for growth chart modeling. To investigate genotype–phenotype relationships, FBN1 variant type was included as an independent variable in height-for-age and BMI-for-age models. MFS-related height increase was compared with that of previous MFS growth studies from the United States, Korea, and France. Height and weight data of 389 individuals with MFS were included (210 males). Height-for-age, BMI-for-age, and weight-for-height charts reflected the tall and slender MFS habitus throughout childhood. Mean increase in height of individuals with MFS compared with the general Dutch population was significantly lower than in the other three MFS populations compared to their reference populations. FBN1-HI variants were associated with taller height in both sexes, and decreased BMI in females (p-values <0.05). This Dutch MFS growth study broadens the notion that genetic background and MFS variant type (HI/DN) influence tall and slender stature in MFS.
1 INTRODUCTION
Marfan syndrome (MFS; OMIM# 154700) is an autosomal dominant connective tissue disorder caused by pathogenic variants in the fibrillin-1 gene FBN1 (OMIM# 134797) with an estimated prevalence of 10.2 per 100,000 individuals (Chiu et al., 2014). It is characterized by skeletal, cardiac and ocular manifestations—for example, tall stature, scoliosis, kyphosis, pectus excavatum, pes planus; aortic root dilation, mitral valve prolapse; myopia, and ectopia lentis (Loeys et al., 2010).
Up-to-date growth charts for both height and weight derived from healthy children and adolescents are essential tools for health workers in preventive and clinical child health care to assess a child's growth. For several genetic syndromes, specific growth charts have been developed. These can be used to plot the patient's growth data to assess if they are consistent with patients with the same syndrome and to detect any aberrant pattern, and to inform patient and parents about expected future growth, including adult height. Three MFS growth charts are available, based on individuals with MFS from France, Korea, and United States (Benoist et al., 2018; Erkula et al., 2002; Kwun et al., 2015); however, Dutch MFS growth charts are currently unavailable. To optimize care for individuals with MFS in the Netherlands, the first goal of this study was to develop Dutch MFS growth charts.
Children in the Netherlands are among the tallest in the world, and are, on average, considerably taller than children from the aforementioned populations (Rodriguez-Martinez et al., 2020). Longitudinal growth in syndromes also varies due to genetic background—for example, individuals with Down syndrome in the Netherlands are up to 10 cm taller than individuals with Down syndrome in other countries (Cremers et al., 1996; Van Gameren-Oosterom et al., 2012). We anticipated that children with MFS in the Netherlands would be taller than their peers from other countries. In addition, it is conceivable that the relative gain in height of individuals with MFS is similar across populations, which would imply that the slopes of MFS-specific growth charts could be transposable to other countries. So, the second goal of this study was to explore the increase in growth attributable to MFS in different reference populations.
It has recently been shown that the types of FBN1 variants—dominant negative (DN) variants (DN effect due to incorporation of abnormal fibrillin-1 in extracellular matrix) and variants leading to haploinsufficiency (HI; loss-of-function due to a reduced amount of fibrillin-1 in extracellular matrix)—to some extent determine the phenotypical differences in individuals with MFS (Franken et al., 2014). Some studies have suggested that the aortic phenotype of adults with HI variants is more severe, though the pathophysiological mechanisms remain unclear (Arnaud et al., 2021; Baudhuin et al., 2015; Franken et al., 2016; Franken et al., 2017) and other studies have not confirmed this (Meester et al., 2022). The only strong genotype–phenotype correlation is the observation that ectopia lentis is more common in individuals with MFS with missense variants, especially those creating or deleting cysteine residues (Arnaud et al., 2021; Faivre et al., 2007; Meester et al., 2022). Therefore, the third goal of this study was to explore whether HI and DN variants have different effects on MFS anthropometrics.
2 MATERIALS AND METHODS
2.1 Editorial policies and ethical considerations
Study procedures were approved by the Medical ethics committee of the Amsterdam University Medical Center (W18_187#18.226). Informed consent was waived as all data were anonymized prior to analysis, and collected by the physicians of the included individuals with MFS.
2.2 Data collection
Longitudinal height and weight data of individuals with MFS, measured at age 0–21 years, were retrospectively collected from all multidisciplinary expert centers for MFS and related disorders in the Netherlands (Amsterdam, Groningen, Leiden, Maastricht, Nijmegen, and Rotterdam).
Inclusion criteria were a diagnosis of MFS according to the 2010 revised Ghent criteria (Loeys et al., 2010) with an established pathogenic FBN1 variant, to prevent inclusion of individuals with MFS-related disorders, such as Loeys-Dietz syndrome. The fourth Dutch growth study charts for healthy children were based on growth measurements from children born between 1976 and 1997. The fifth (currently employed) growth study charts in the Netherlands did not show a secular trend compared to the fourth Dutch growth study charts (Fredriks et al., 2000; Schonbeck et al., 2013; Talma et al., 2010). Thus, only data of individuals born after January 1, 1976 were included, to prevent adjusting for the positive secular trend that was present in the Netherlands before the fourth Dutch growth study (Fredriks et al., 2000).
Exclusion criteria were scoliosis >25°, kyphosis >60°, leg length discrepancy >3 cm, and a concurrent condition affecting height or weight. Growth data documented after surgical care for scoliosis or kyphosis, or after adult height diminishing procedures as bilateral knee epiphysiodesis and supraphysiological sex hormone treatment were also excluded. Growth data were only included if a minimum of 3 months had passed in between two measurements.
Other information that was collected included birth year and month, gender, FBN1 variant, and the ethnic origin of the individual (Dutch descent or non-Dutch descent). To control for possible double data entry in case an individual was treated in two centers, we reviewed FBN1 variant, sex, birth year, and month of all individuals.
2.3 Variant classification
Pathogenicity of FBN1 variants was determined according to the American College of Medical Genetics and Genomics standards and guidelines for the interpretation of sequence variants (Richards et al., 2015). All variants were pathogenic or likely pathogenic. FBN1 variants were classified as HI or DN by one expert (GP) according to criteria previously described (Franken et al., 2015). In the case of uncertainty on FBN1 variant type, no type was assigned. All identified FBN1 variants are given in Supplementary Table 1, together with FBN1 variant type (HI/DN). FBN1 transcript NM_000138.4 was used to annotate the variants. An exon numbering was used where exon 1 contains the start codon—and exon 0 is the untranslated 5′UTR exon.
2.4 Statistical analysis
Growth data curation was performed by plotting the height-for-age and weight-for-age charts of each individual, to check for inconsistencies.
The Generalized Additive Models for Location, Scale and Shape method—implemented in R as the gamlss package—was used for growth chart modeling (Rigby & Stasinopoulos, 2005). We implemented the untruncated Box-Cox t distribution to model growth charts. This continuous distribution encompasses four parameters involving the median (50th centile), variation, skewness, and kurtosis. The four parameters were allowed to vary over age using p-splines (Stasinopoulos & Rigby, 2007). Modeling was performed in R version 4.1.0. There were not enough height and weight measurements collected at ages <1 year and >18 years for an accurate model, so these measurements were filtered from the dataset and all analyses. Data of males and females were analyzed separately. The following growth charts were constructed: height-for-age, weight-for-age, weight-for-height, and BMI-for-age. The growth charts were fitted with centile lines corresponding to the 2.3th, 15.9th, 50th, 84.1th, and 97.7th percentile, conform the Dutch reference growth charts of 2010 (Talma et al., 2010), and corresponding to −2, −1, 0, +1, and +2 SDS. To investigate the effect of (1) FBN1 variant type and (2) genetic background (population) on height and BMI, models were fitted including FBN1 variant type and ethnicity as variables in the model formula.
Mean final height (also termed adult height) of individuals with MFS from the current study and previous MFS growth studies—defined as the mean height recorded at the oldest age in the MFS growth studies (United States, 20 years; Korea, 20 years; France, 17 years; and the Netherlands, 18 years (Benoist et al., 2018; Erkula et al., 2002; Kwun et al., 2015)—were expressed as HSDS (compared to the respective reference populations at the age final height was recorded in the MFS growth studies (Kuczmarski et al., 2002; Moon et al., 2008; Talma et al., 2010)). So for example, mean height of Dutch individuals with MFS at 18 years was compared to the Dutch reference population at 18 years, and expressed as HSDS. Then, to analyze the effect of genetic background (country of origin) on MFS anthropometrics, we compared the Dutch MFS final height HSDS with the MFS final height HSDS of each previous MFS growth study, using the unpaired t test. For the French MFS growth study, we used the 2007 WHO growth standards as reference, since this fits the French population better than the 1974 growth standards (Benoist et al., 2018). Statistical analyses were performed using R version 4.1.0.
3 RESULTS
3.1 Individuals
1058 height measurements and 995 weight measurements of 210 males, and 891 height measurements and 831 weight measurements of 179 females were used for data analysis and construction of growth charts. Median follow-up in years was 4.9 (range 0–16.0) for males and 5.1 (range 0–14.6) for females. Median number and range of measurements was as follows: height measurements in males, 5.0 (1–15); height measurements in females, 5.0 (1–14); weight measurements in males, 4.9 (1–13); weight measurements in females, 4.7 (1–14). 82.8% of the individuals were native Dutch (both parents born in the Netherlands). FBN1 variants were classified as DN in 59.4% of the individuals and as HI in 35.0% (in 5.6% of the individuals there were missing or incomplete data on the specific FBN1 variant).
To assess the impact of including individuals of non-Dutch descent in our dataset, height-for-age models were fitted including ethnicity as an independent variable. Non-Dutch ethnicity was associated with a lower mean height for males (p-value = 7.77e-06). The addition of individuals with a non-Dutch ethnicity decreased mean height at the age of 18 years from 195.8 cm to 195.3 cm. In females, ethnicity did not have a significant association with mean height (p-value = 0.317), and the addition of individuals with a non-Dutch ethnicity increased mean height at the age of 18 from 180.9 to 181.0 cm. As the mean height was marginally different when including the non-Dutch descent group, we chose to not exclude these individuals.
3.2 Dutch growth charts
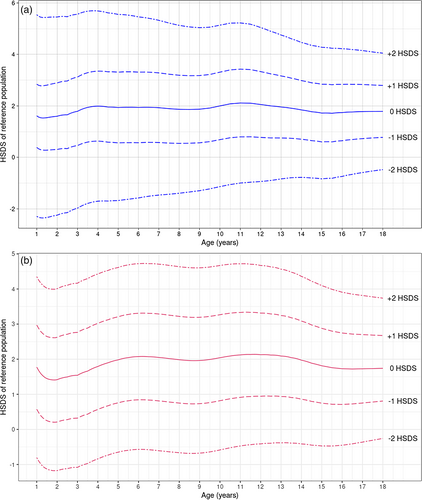
Consistent with previous reports on linear growth in MFS, both males and females with MFS showed increased height compared with the reference population throughout infancy, childhood, and adolescence (Figure 1a,b). The linear growth curves leveled in males at the age of 15 and in females at the age of 14, as in the reference populations. Thus, enhanced height in MFS is not due to prolonged growth, but due to enhanced growth rate only. Mean HSDS of individuals with MFS coincided with +2 SDS at all ages and for both sexes. To visualize this more clearly, HSDS-for-age charts were prepared, showing mean, ±1 and ±2 HSDS of individuals with MFS (inferred from the growth centiles) compared to the reference population (Figure 2a,b).
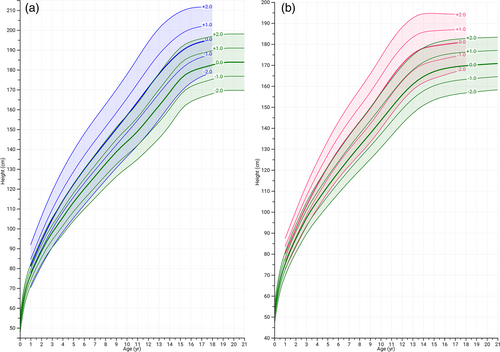
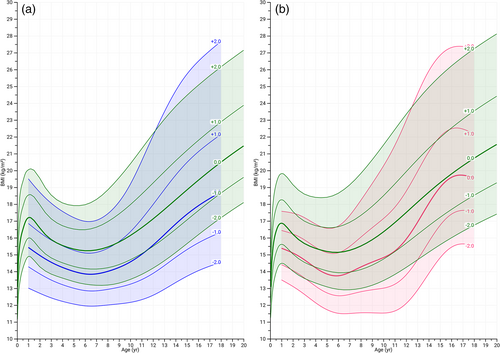
Overall, in male and female individuals with MFS, the mean BMI is reduced before the age of 12 as compared to the general population, after which BMI became more variable in MFS. The mean BMI trajectory of males with MFS throughout the growth phase followed the −1 SDS of the reference population, whereas in females BMI gradually increased from −1 SDS to 0 SDS during puberty (Figure 3a,b).
Throughout the height distribution, most individuals with MFS had a lower weight-for-height than the mean value in the reference population, following the −1 SD curve (Supplementary Figure 1a,b). Weight-for-age charts were comparable with reference population charts, and mean weight-for-age was even somewhat higher (+0.5 to +1 SD) in childhood and adolescence (Supplementary Figure 2a,b).
3.3 Comparison of MFS final height SDS per population
To study the effect of genetic background (country of origin) on MFS anthropometrics, we compared final heights of previous MFS growth studies with the current study (Tables 1 and 2). Although mean final height in the Dutch individuals with MFS was greater than in all other previously studied populations, the MFS-related increase in mean final height (expressed as a HSDS of the respective reference population) was significantly lower than previously reported in other cohorts (Tables 1 and 2). An exception was comparison of female individuals with MFS of the Dutch and US cohorts, which were similar. The post hoc statistical power of all comparisons was >80% except for Dutch males versus US males (73.1% power) and Dutch females vs. US females (18% power) due to the low number of female subjects in the US dataset.
| Study population | Study sample | Molecularly confirmed MFS | Mean final height ± SD of individuals with MFSa | Mean final height ± SD of reference populationb | MFS mean final height expressed as HSDS of reference population ± SD (p-valuec) |
|---|---|---|---|---|---|
| United States, 2002 (Erkula et al., 2002) | 99 males | No | 191.3 ± 9 cm | 176.7 ± 7.1 cm | 2.1 ± 1.3 (0.0406) |
| Korea, 2015 (Kwun et al., 2015) | 187 males | No | 191.5 ± 5.3 cm | 173.4 ± 5.6 cm | 3.2 ± 0.9 (<0.0001) |
| France, 2018 (Benoist et al., 2018) | 134 males | Yes | 191.2 ± 8.4 cm | 175.2 ± 7.6 cm | 2.1 ± 1.1 (0.0053) |
| The Netherlands, this study | 210 males | Yes | 195.3 ± 7.3 cm | 182.4 ± 7.3 cm | 1.8 ± 1.0 |
- Abbreviation: MFS, Marfan syndrome.
- a Final height was here defined as height recorded at the oldest age in the MFS growth studies (United States, 20 years; Korea, 20 years; France, 17 years; and the Netherlands, 18 years).
- b Mean height of the reference population is shown at the same age as given above, for each population.
- c Mean final height expressed as HSDS of previous MFS growth studies and the current study were compared using the unpaired t test.
| Study population | Study sample | Molecularly confirmed MFS | Mean final height ± SD of individuals with MFSa | Mean final height ± SD of reference populationb | MFS mean final height expressed as HSDS of reference population ± SD (p-valuec) |
|---|---|---|---|---|---|
| United States, 2002 (Erkula et al., 2002) | 81 females | No | 175.4 ± 8.2 cm | 163.1 ± 6.5 cm | 1.9 ± 1.3 (0.2795) |
| Korea, 2015 (Kwun et al., 2015) | 152 females | No | 176.2 ± 5.4 cm | 160.7 ± 5.0 cm | 3.1 ± 1.1 (<0.0001) |
| France, 2018 (Benoist et al., 2018) | 125 females | Yes | 178.3 ± 7.6 cm | 162.8 ± 6.6 cm | 2.3 ± 0.7 (<0.0001) |
| The Netherlands, this study | 179 females | Yes | 181.0 ± 6.0 cm | 169.7 ± 6.2 cm | 1.7 ± 0.9 |
- Abbreviation: MFS, Marfan syndrome.
- a Final height was here defined as height recorded at the oldest age in the MFS growth studies (United States, 20 years; Korea, 20 years; France, 17 years; and the Netherlands, 18 years).
- b Mean height of the reference population is shown at the same age as given above, for each population.
- c Mean final height expressed as HSDS of previous MFS growth studies and the current study were compared using the unpaired t test.
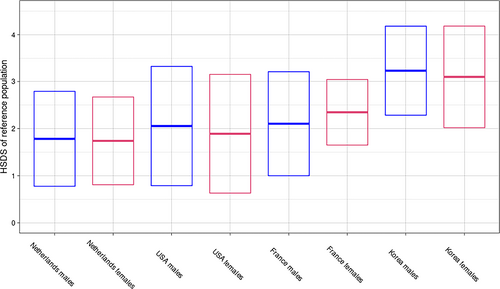
We further plotted mean final height of individuals with MFS across populations, to visualize the effect of ethnicity on MFS anthropometrics (Figure 4). Notably, individuals with MFS in taller populations attain a relatively shorter final height compared to the reference population than in shorter populations (Figure 4).
3.4 Effect of FBN1 variant type on MFS anthropometrics
The effect of HI and DN FBN1 variants on linear growth and BMI in MFS was investigated by including FBN1 variant type as an independent variable in height-for-age and BMI-for-age models. At 18 years of age, mean height was taller in individuals with an HI variant compared with those with a DN variant: 196.1 cm versus 195.0 cm in males (p-value = 0.000298), and 181.8 cm versus 180.7 cm in females (p-value = 0.00285). Considering BMI, only in females we found a significant association with FBN1 variant type (p-value = 0.027); however, the actual difference between mean BMI values at age 18 years was very small: 19.9 kg/m2 in individuals with a DN variant, and 19.7 kg/m2 in individuals with an HI variant.
4 DISCUSSION
To optimize pediatric care for individuals with MFS in the Netherlands, we retrospectively collected anthropometric and genetic data of individuals with MFS aged 0–21, from all multidisciplinary expert centers for MFS in the Netherlands. We constructed MFS growth charts, including height-for-age, BMI-for-age, weight-for-height and weight-for-age charts. We compared linear growth of Dutch individuals with foreign MFS cohorts and found that the increase in HSDS is not equal across different populations. While the Dutch MFS population was the tallest among the different MFS populations, the relative gain in height of individuals with MFS in the Netherlands was significantly lower than previously reported in other populations. Further, we provide evidence for genotype–phenotype relationships, as FBN1 HI variants were significantly associated with taller height (p-value <0.01) in both males and females. Also, a significant association of FBN1 HI variant with lower BMI in females (p-value = 0.027) was found.
Growth charts based on population reference studies are valuable tools for assessing growth in children in preventive and curative child health care, and identifying short or tall stature (or faltering or accelerating growth) which should lead to further diagnostic work-up (Lauffer et al., 2019; Wit et al., 2019). Moreover, assessment of the individual growth curve plotted on height-for-age charts in combination with bone age analysis permits prediction of adult height and thereby facilitates decision-making concerning epiphysiodesis in the case of extremely tall predicted stature (Goedegebuure et al., 2018; Rozendaal et al., 2005). Nevertheless, pediatricians are frequently faced by the conundrum of growth evaluation in syndromic patients, because syndrome-specific growth charts are only available for a small number of syndromes. Also, since genetic background (e.g., ethnicity) greatly influences anthropometrics, syndrome-specific growth charts may be unvalid, if they are based on smaller or taller general populations. We expect that medical care of children with MFS in the Netherlands will improve with the implementation of our MFS growth charts, because plotting growth data on these charts will provide information on whether the patient's growth pattern is consistent with that of other individuals with the same syndrome, and allows a rough prediction of future growth.
Reviewing growth patterns of MFS across different populations (Tables 1 and 2, Figure 4), it seems that Dutch individuals with MFS tend to be relatively less tall compared to the reference populations in the previously published Korean, United States, and French cohorts (Benoist et al., 2018; Erkula et al., 2002; Kwun et al., 2015). The observed heterogeneity in MFS mean final heights could be instigated by the effects of particular genetic backgrounds on MFS anthropometrics. Similarly, it has been reported that the genetic background of a MFS mouse model influenced length (Lima et al., 2010). Moreover, it was shown that several aspects of the MFS phenotype differed significantly between MFS populations comparing individuals of Asian and Caucasian descent (Franken et al., 2013). Thus, we propose that the increase in HSDS in MFS for a particular genetic background depends on a complex interplay of genetic and environmental factors. An important implication of the notion that the increase in HSDS of individuals with MFS is not equal across populations, is that this impedes transposing MFS growth charts to other countries (without checking height of the local population).
We speculate that the heterogeneity in MFS mean final heights may be linked to the increased height of the general Dutch population (Stulp et al., 2015; Zhong et al., 2017). Hypothetically, the excessive bone growth process in MFS partially overlaps with the transforming growth factor beta (TGFβ) family-involved natural bone growth process in the general population (Jianwei et al., 2022; Oton-Gonzalez et al., 2022), weakening the MFS-related increase in HSDS. It is known that in MFS the TGFβ signaling pathways are disturbed (Dawson et al., 2021; Le Goff & Cormier-Daire, 2012; Ramirez et al., 2018). Recently, it was shown that fibrillin-1 deficient perichondrium caused disturbed TGFβ signaling and thus enhanced chondrogenesis in the neighboring epiphyseal growth plate in MFS mice, causing the long bone overgrowth (Sedes et al., 2022).
Results from our analysis of the effect of FBN1 variant type on MFS height and BMI charts provide novel insight in the MFS phenotype, as we found significant associations between genotype (FBN1 HI/DN variant) and MFS anthropometrics. The finding of increased linear growth and decreased BMI in individuals with an HI variant aligns with the notion of MFS genotype–phenotype correlations. Interestingly, it was previously reported that skeletal features of MFS (including tall stature) were more prominent in individuals with an HI variant (Arnaud et al., 2021; Franken et al., 2014), which is in line with our results on growth in MFS.
The classification of HI and DN is a prediction in most cases, thus FBN1 variant outcome may be surprising. As an example of unexpected findings, exon skipping of exon 52 in 50% of the fibrillin-1 mRNA resulted in complete loss of fibrillin-1 fiber formation in the extracellular matrix, while fibers reappeared when >80% of exon skipping was achieved, revealing that a mismatch in size of the fibrillin-1 protein may hamper fibrillin-1 fiber formation (Cale et al., 2021). Thus, among the FBN1 DN variant type, tall individuals may indeed have reduced fibrillin-1 fiber formation (HI phenotype), while producing the fibrillin-1 protein. This needs to be further explored before height or weight can be used as proxy for fibrillin-1 fiber abundance.
As for the association between decreased BMI and HI variant in females, we speculate that it may be related to the C-terminal tail of the FBN1 gene, which contains the coding sequence of the asprosin hormone peptide. Asprosin is cleaved from the fibrillin-1 protein and regulates metabolism, appetite and adipogensis (Duerrschmid et al., 2017; Muthu & Reinhardt, 2020; Romere et al., 2016). Individuals with pathogenic variants in this part of the FBN1 gene do not have MFS but Marfanoid-progeroid-lipodystrophy syndrome (OMIM# 616914), characterized by defective adipogenesis (Passarge et al., 2016). In theory, MFS patients with reduced fibrillin-1 protein production (HI phenotype) may have a mild form of asprosin deficiency and may thus have altered metabolism and adipogenesis, and consequently a lean appearance with reduced BMI. However, this has yet to be proven in the FBN1 HI variant type in MFS. Future study of other aspects of the MFS phenotype in relation to FBN1 variant type are likely to further clarify MFS phenotypical heterogeneity and etiopathogenesis.
This study presents some limitations. First, selection bias is a potential concern since MFS is possibly an underdiagnosed condition in childhood, due to the apparent mildness of the MFS phenotype in some individuals (Lauffer et al., 2019). Furthermore, by omitting data of individuals recorded after epiphysiodesis or hormone therapy, we may have missed the tallest measurements of children in the datasets. Second, due to the retrospective nature of this study, we could not influence the number of measurements per patient and quality of measurements, and were not able to collect bone age assessments and parental heights in a structured way. Third, since there were not enough measurements collected at ages <1 and >18, we could not model the MFS growth charts beyond these ages.
The strengths of this study include that the analysis is based on nationwide data, and that solely individuals with MFS and a pathogenic FBN1 variant were included—to minimize the risk of including individuals with, for example, MFS-like syndromes as Loeys-Dietz syndrome.
In summary, we present the first Dutch MFS syndrome-specific growth charts. Since growth in syndromes varies due to genetic background, other available MFS growth charts are unsuitable for the use in the Netherlands, the tallest population in the world. Using nation-wide growth data, we created Dutch MFS growth charts, including height, weight and BMI-for-age charts, and weight-for-height charts. The generated data present interesting insights in the MFS phenotype. Our comparison of growth in MFS across populations with different ethnic backgrounds revealed that the increase in HSDS of individuals with MFS is not equal across populations, which impedes transposing MFS growth charts to other countries. In addition, our data indicate that MFS genotype is related to anthropometrics—specifically HI FBN1 variants were associated with taller height (in males and females) and lower BMI (in females), compared to DN FBN1 variants. Taken together, our results broaden the notion of associations between MFS genotype and other genetic factors (e.g., genetic background), and phenotypical variability in MFS.
AUTHOR CONTRIBUTIONS
Data acquisition: Peter Lauffer, Floor A. M. Postema, Jessica Warnink-Kavelaars, Eelco Dulfer, Yvonne Hilhorst-Hofstee, Marlies Kempers, Ingrid P. C. Krapels, Ingrid M. B. H van de Laar, Bart Loeys, Arjan C. Houweling, and Marieke J. H. Baars. FBN1 variant classification: Gerard Pals. Statistical analyses: Peter Lauffer, Alexander M. J. Spaans, and Aeilko H. Zwinderman. Writing of first draft: Peter Lauffer and Leonie A. Menke. Revision of intellectual content: Vivian de Waard and Jan M. Wit. Project design and coordination: Leonie A. Menke. All authors critically reviewed manuscript drafts and approved the final manuscript as submitted.
ACKNOWLEDGMENTS
The authors thank Annelies van der Hulst, pediatric-cardiologist; Elke Kraal-Biezen, ophthalmologist; Madeleine Tilburgs, pediatric assistant; and Alessandra Maugeri, molecular geneticist, for their important roles in the Amsterdam Expert Center for children with Marfan syndrome and related disorders.
FUNDING INFORMATION
This study was supported by grants of the Dutch patient association “Contactgroep Marfan Nederland” and the Emma Children's Hospital Foundation (WAR2017-24).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Variant data were submitted to a public database (ClinVar).




