A de novo hexokinase 1 (HK1) variant presenting as Boucher–Neuhäuser syndrome
Funding information: National Human Genome Research Institute; National Institutes of Health
Abstract
Boucher–Neuhäuser syndrome (BNHS) is characterized by chorioretinal dystrophy, hypogonadotropic hypogonadism, and cerebellar dysfunction and atrophy. The disorder has been associated with biallelic pathogenic variants in the patatin-like phospholipase domain-containing protein 6 (PNPLA6) gene. We present an individual with a clinical diagnosis consistent with BNHS who lacked any PNPLA6 variants but on quartet family exome sequencing had a de novo variant in the hexokinase 1 (HK1) gene (NM_000188.2 [GRCh37/hg19]: g.71139826G>A, c.1240G>A, p.Gly414Arg), suggesting genetic heterogeneity for BNHS. Longitudinal follow-up indicated neurological deterioration, neuropsychiatric symptoms, and progressive cerebellar atrophy. The BNHS phenotype overlaps and expands the known HK1 genotypic and phenotypic spectrum. Individuals with variants in HK1 should undergo evaluation for hypogonadotropic hypogonadism, potentially amenable to treatment.
1 INTRODUCTION
Boucher–Neuhäuser syndrome (BNHS, OMIM #215470) is a disorder characterized by the triad of chorioretinal dystrophy, hypogonadotropic hypogonadism, and cerebellar dysfunction and ataxia. BNHS is associated with biallelic pathogenic variants in the patatin-like phospholipase domain-containing protein 6 (PNPLA6) gene on chromosome 19p13.2 and exhibits autosomal recessive inheritance (Boucher & Gibberd, 1969; Limber et al., 1989; Neuhäuser & Opitz, 1975; Synofzik et al., 2014). In addition to the characteristic symptom triad, BNHS may also include other neurological abnormalities such as pyramidal tract signs, peripheral neuropathy, cognitive dysfunction, and atrophy of the cerebellum and brainstem (Tarnutzer et al., 2015). Just over 65 cases of biallelic PNPLA6 variants have been reported, suggesting the rare prevalence of this syndrome (Synofzik et al., 2021; Wu et al., 2021).
Hexokinase 1 (HK1) is a gene on chromosome 10q22.1 that catalyzes the phosphorylation of glucose to glucose-6-phosphate using adenosine triphosphate in glycolysis (Griffin et al., 1991). Mutations in HK1 have been associated with four phenotypes: autosomal dominant (AD) neurodevelopmental disorder with visual defects and brain anomalies (NEDVIBA, OMIM #618547; Okur et al., 2019); AD retinitis pigmentosa 79 (RP79, OMIM #617460; Sullivan et al., 2014); autosomal recessive (AR) Russe type hereditary motor and sensory neuropathy (HMSNR, OMIM #605285; Hantke et al., 2009); and AR nonspherocytic hemolytic anemia (OMIM #235700; Jamwal et al., 2019).
The association of a BNHS phenotype with monoallelic HK1 has not previously been made. We report a male individual with the BNHS phenotype who, on quartet family exome sequencing, had a de novo HK1 variant but no variant in PNPLA6. This expands the HK1 phenotypic spectrum and the BNHS genotypic spectrum.
2 CLINICAL REPORT
2.1 Editorial policies and ethical considerations
The patient and his family were evaluated under the auspices of the National Institutes of Health (NIH) Undiagnosed Diseases Program (UDP; Gahl & Tifft, 2011; Gahl et al., 2012; Gahl et al., 2016). The study, clinical protocol 76-HG-0238, “Diagnosis and Treatment of Patients with Inborn Errors of Metabolism and Other Genetic Disorders,” was approved by the NIH National Human Genome Research Institute (NHGRI) Institutional Review Board, and the patient provided written consent for publication.
2.2 Case report
The patient was born full term to non-consanguineous parents without complications, illnesses, or exposures. During childhood, he was considered uncoordinated and unable to hop on one foot or catch a ball but able to ride a bicycle. He completed culinary school at age 22 and transitioned to independent living. He struggled with anxiety, forgetfulness, and grooming and exhibited increased tripping and depressive symptoms. He began to lose his ability to ride his bicycle. Following a fall, he was found to have decreased vision. Brain magnetic resonance imaging (MRI) was normal, but he was declared legally blind by age 23 and given a presumptive diagnosis of rod-cone dystrophy. He also began experiencing behavioral regression. An electroencephalogram and gene panels evaluating for spinocerebellar ataxia and mitochondrial gene variants were all uninformative.
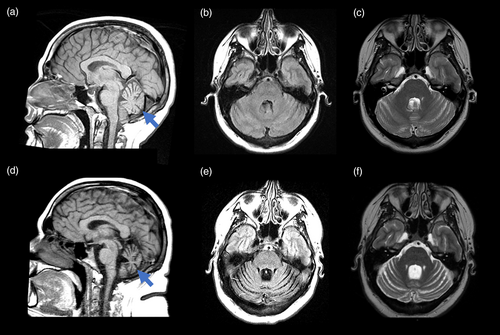
At the time of our evaluation at 24 years of age, additional symptoms included fatigue and somnolence. He was obese, with a body mass index of 40.6 kg/m2 and a height of 167 cm with lower abdominal striae. He had blue eyes, lightly pigmented skin, and mild iris transillumination. There was slight rounding of the face with mild facial plethora. His speech was slightly muffled but understandable. Swallowing was normal. He exhibited slow movements, mild end-point intention tremor (less than 2.0 cm in amplitude) on finger-to-nose test, mild dysmetria, a trace of rigidity with reinforcement in the upper extremities, minimal choreoathetoid movements of his upper extremities, and shortening of the Achilles' heels. His base of support when standing was approximately one foot wide, and his gait was characterized by decreased arm swing, imbalance, and mild stooped posture. He was unable to tandem walk or stand on one foot unassisted. He had a positive retropulsion test and negative Romberg test. Sensory exam was normal. He had 3 cm of gynecomastia bilaterally and a slightly small penis with normal testicle volume. Neuropsychological testing noted mild impairment in executive functioning and attention with increased apathy. Magnetic resonance imaging (MRI) of the brain showed a small, homogenous pituitary gland of 3 mm in vertical diameter (reference range for age and sex is 6.6 ± 1.5 mm standard deviation [Yadav et al., 2017]) with mild cerebellar atrophy (Figure 1). Magnetic resonance spectroscopy revealed low N-acetylaspartate level in the superior cerebellar vermis. Over three consecutive days, the total testosterone level ranged from 189 to 231 ng/L, which was low (reference range of 240–950 ng/L), with a free testosterone level ranging from 5.7 to 8.3 ng/L, which was also low (reference range 9–30 ng/L). Sex hormone binding globulin, follicle stimulating hormone, luteinizing hormone, serum estrone, thyroid stimulating hormone, free thyroxine, triiodothyronine, prolactin, adrenocorticotropic hormone, cortisol, insulin-like growth factor-1 (IGF-1), and growth hormone were normal compared to reference ranges (Supporting Information).
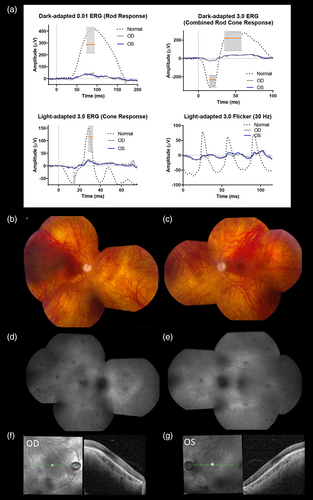
Eye exam revealed compound astigmatism with high myopia and best corrected visual acuity of 20/50 in each eye. He reported night vision difficulties, sensitivity to light, reduced peripheral vision, and reduced color vision. Ophthalmologic examination demonstrated full extraocular muscle movement in all planes but with rare “square wave” jerks in neutral gaze and saccadic intrusion during smooth pursuit in all planes with mild saccadic dysmetria. Nystagmus was not present in any direction of gaze and was also absent in end-gaze. Retinal degeneration and optic atrophy were present (Figure 2). Optical Coherence Tomography (OCT) scans of the macula showed grade 1 foveal hypoplasia with intact central photoreceptors. Retinal nerve fiber layer (RNFL) and ganglion cell layer thinning were identified. Full-field electroretinography (ERG) revealed bilateral severe diffuse outer retinal dysfunction affecting rods more than cones in each eye, characterized by reduction of scotopic responses by about 80%–85% below the lower limits of normal in amplitude with normal timing in both eyes. Photopic responses were reduced by approximately 70% below the lower limits of normal in amplitude with delayed timing in both eyes (Figure 2). The macula was relatively preserved. At 31 years of age, Goldmann visual field, full-field ERG, and spectral domain optical coherence tomography (OCT) revealed only progressive reduction of cone-mediated responses. At 34 years of age, he had additional difficulty adjusting from dark into light and from light into dark conditions without significant change in full-field ERG or OCT.
Quartet family exome sequencing (Illumina TruSeqV1), which included the patient, biological parents, and older sister, revealed that the patient carried a de novo variant in the hexokinase 1 (HK1) gene (GRCh37/hg19) NM_000188.2: g.71139826G>A, c.1240G>A, p.Gly414Arg. This variant was confirmed by Sanger sequencing (GeneDx, Gaithersburg, Maryland). Known disease-causing variants were not identified in any other disease-associated gene, including PNPLA6. A high-density single nucleotide polymorphism (SNP) array (HumanOmniExpress-12 v1.0, Illumina, San Diego, CA) did not indicate large structural abnormalities in PNPLA6 or HK1. We determined this variant of interest in HK1 to be likely pathogenic using the following criteria: PS2, PM2, PM5, PP2, PP3, and without functional data for this variant (Supporting Information; Richards et al., 2015).
A whole blood O-glycan profile (Emory Genetics Laboratory, Decatur, GA) for congenital disorders of glycosylation found a significant elevation of T antigen (2.19 μmol/L, reference 0.22–1.14 μmol/L) with an increased T antigen/sialyl-T antigen ratio (0.11, reference <0.06) by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry. MALDI-TOF mass spectrometry also determined an increased ratio between monosialyl core 2/disialyl core 2, which may indicate hyposialylation in O-linked glycans. N-linked glycan analysis revealed borderline decreases in sialylation in N-linked glycans in a nonspecific pattern. These results are concerning for a disorder of glycosylation.
Follow-up MRI testing at age 33 revealed progression to generalized cerebellar atrophy with involvement of the posterior lobule without cerebral involvement (Figure 1). At 35 years of age, he experienced increasing balance issues with frequent falls and prominent psychiatric symptoms including an episode of mania occurring after exposure to SSRI therapy for anxiety, panic attacks, agoraphobia, and rare hallucinations. The compounding effects of his physical, cognitive, and psychiatric symptoms contributed to difficulty living independently.
3 DISCUSSION
We describe the association of a HK1 (hexokinase 1) variant with a BNHS phenotype. This individual has progressive cerebellar deficits and cerebellar atrophy, retinal degeneration, and hypogonadotropic hypogonadism with a small pituitary gland that necessitated treatment with testosterone injections. Additional findings included optic atrophy, apathy, mood changes, impairment in executive functioning, and mild parkinsonism.
Another diagnosis with cerebellar ataxia and hypogonadotropic hypogonadism is Gordon Holmes syndrome (GDHS, OMIM #212840), but the individual we report here does not have known pathogenic variants in RNF216 or have findings of cerebral atrophy or dysarthria. Also, 4H syndrome (OMIM #607694) was considered, but our patient has normal dentition and preserved myelination without leukodystrophy or pathogenic variants in POLR3A.
HK1 is a major hexokinase isozyme found in the brain and photoreceptors and located on the outer mitochondrial membrane (OMM; Sullivan et al., 2014). This individual's follow-up brain MRI at 33 years of age revealed progression of atrophy extensively involving the posterior lobule of the cerebellum, which has been associated with cerebellar cognitive affective syndrome that might account for at least part of his cognitive and behavioral symptoms (Schmahmann & Sherman, 1998). Bioenergetic deficits have been implicated in the pathogenesis of primary psychiatric disorders, and decreased HK1 protein has been uncovered in brain samples of patients with unipolar depression, bipolar disorder, and schizophrenia (Regenold et al., 2012).
We report a single individual, but a similar case has been published within a series of patients having a neurodevelopmental phenotype associated with de novo HK1 variants. Specifically, a 34-year-old woman with a de novo variant at the same amino acid location (HK1 c.1241G>A, p.Gly414Glu) overlapped phenotypically with our patient, including motor developmental delays, ataxia, and anxiety, though that individual developed a different ocular phenotype of retinitis pigmentosa and peripheral vision loss (Okur et al., 2019). Endocrine abnormalities were not mentioned, but progression of symptoms was suggested for several patients in the case series. Hexokinase enzyme activity was reported normal in their cases, suggesting that a mechanism beyond the canonical HK1 enzyme role might be responsible for the molecular pathology. Disease mechanism and rate of progression remain uncertain. Our patient had a unique glycosylation and sialylation pattern suggesting that HK1 variants might have a yet uncharacterized effect on the protein glycosylation process, with pathophysiological implications. Anti-apoptotic regulatory properties of HK1, in conjunction with tumor necrosis factor via pro-inflammatory mechanisms, could also mediate aspects of disease progression (Schindler & Foley, 2013).
ACKNOWLEDGMENTS
We would like to thank this patient for allowing this publication and the healthcare providers who have been involved in his care. This work was supported in part by the Intramural Research Program of the National Human Genome Research Institute at the National Institutes of Health.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available within the context of this article.




