The clinical features of OSTM1-associated malignant infantile osteopetrosis: A retrospective, single-center experience over one decade
Abstract
Mutation in OSTM1 give rise to the rarest and most lethal subtype of malignant infantile osteopetrosis (MIOP), and an improved understanding of OSTM1-associated MIOP would help with informed decision-making regarding symptom management and early palliative care referral. This retrospective study describes the clinical and laboratory features of patients with a genetic diagnosis of OSTM1 MIOP made between January 2011 and December 2021 in the Department of Pediatrics, Al-Adan Hospital, Kuwait. Twenty-two children had confirmed homozygous deletion in OSTM1 (13 females, nine males). Consanguinity was reported in almost all parents. 72.7% were diagnosed before the age of two months, most commonly incidentally with a high clinical suspicion. All 22 patients developed upper respiratory symptoms, hepatosplenomegaly, poor feeding, and had severe developmental delay. 80% of patients developed pain and/or irritability, and 40.9% were diagnosed with primary seizures. Bone fractures developed in 27% of patients, most likely iatrogenic, and some patients had hernia and gum abnormalities. The mean survival was 10.9 months. The clinical presentation, symptomatology, and mortality of our cohort were compared with other cases of OSTM1 MIOP identified through a comperhensive search of the PubMed database. The findings conclude that OSTM1 MIOP is a multi-systemic disease with distinct clinical features, of which neurological complications are the most severe and include nociplastic pain and irritability. Although orthopedic complications influence the trajectory of most patients with other forms of osteopetrosis, OSTM1 MIOP is driven by its neurological complications. Hence, OSTM1 should be regarded as a neurodegenerative disease with osteopetrosis as a comorbidity that warrants early palliative care referral.
1 INTRODUCTION
Malignant infantile osteopetrosis (MIOP) describes a group of severe, rare, autosomal recessive diseases characterized by failure of osteoclast function. The consequent increase in bone density, which starts early in life, causes childhood death if untreated (Balemans et al., 2005; Mazzolari et al., 2009; Sobacchi et al., 2013; Stark & Savarirayan, 2009; Villa et al., 2009). Several genes have been implicated in MIOP including TCIRG1, CLCN7, SNX10, TNFRSF11A (RANK), TNFSF11 (RANKL), and OSTM1 (Balemans et al., 2005; Mazzolari et al., 2009; Sobacchi et al., 2013; Stark & Savarirayan, 2009; Villa et al., 2009). Patients with MIOP can develop bone fractures, short stature, macrocephaly, frontal bossing, choanal stenosis, tooth eruption defects, and narrow nerve foramina resulting in blindness, deafness, and facial palsy. Furthermore, bone marrow suppression results in pancytopenia and hepatosplenomegaly (Sobacchi et al., 2013; Villa et al., 2009).
Some MIOPs are neuropathic variants, including OSTM1 mutations, with a variable clinical picture of significant neurological abnormalities including calcium-independent seizures, developmental delay, hypotonia, retinopathy, and sensorineural deafness (Frattini et al., 2003; Mazzolari et al., 2009; Sobacchi et al., 2013; Villa et al., 2009). OSTM1 mutation-associated MIOP is therefore distinct from the other forms. In particular, the neurodegenerative complications are more severe than the orthopedic complications and the very severe central nervous system involvement is marked by cerebral atrophy and loss of myelination (Heraud et al., 2014; Mazzolari et al., 2009; Ott et al., 2013; Sobacchi et al., 2013; Vacher et al., 2020; Wu et al., 2017). Furthermore, OSTM1 mutation-associated MIOP is more lethal than the other forms, and children with the disease do not survive beyond 2 years of age (Balemans et al., 2005; Heraud et al., 2014; Mazzolari et al., 2009; Ott et al., 2013; Sobacchi et al., 2013; Vacher et al., 2020; Villa et al., 2009; Wu et al., 2017). Hematopoietic cell transplantation (HCT) is curative for many types of MIOP because osteoclasts are of hematopoietic origin, and HCT treatment prevents osteoclast-related manifestations when initiated early (Frattini et al., 2003; Mazzolari et al., 2009; Sobacchi et al., 2013; Stark & Savarirayan, 2009). However, it is not recommended for OSTM1 MIOP because it does not improve outcomes in subtypes of osteopetrosis associated with primary rather than compressive neuropathy (Heraud et al., 2014; Mazzolari et al., 2009; Sobacchi et al., 2013; Villa et al., 2009).
While OSTM1 MIOP is a rare genetic condition globally, it is more common in Kuwait and surrounding areas due to a high prevalence of consanguinity within tribal families. Definitive treatment of the condition is elusive at present. Children with this condition have multiple complex and distressing symptoms. In addition, patients are at risk of iatrogenic complications, as described below. An improved understanding of the clinical presentation of MIOP will help physicians proactively treat symptoms and make better treatment decisions for their patients.
Here, we summarize the clinical and laboratory features of 22 patients (13 females, 9 males) diagnosed with OSTM1 MIOP between 2011 and 2021 at the Department of Pediatrics, Al-Adan Hospital, Kuwait.
2 CASE REPORTS
The clinical features of 22 patients with OSTM1 MIOP are summarized in Table 1. Patient 21 was reported previously (Alotaibi & Dighe, 2021).
| Patient | Gender | Family history | Consanguinity | Age at diagnosis** | Presenting symptom | Failure to thrive | Developmental delay | Pain or irritability | Muscle tone | Primary seizures | Upper airway abnormalities | Feeding difficulties | Hepatospl-enomegaly | Fractures | Soft tissue swelling | Bleeding | Other | Age at death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | F | − | + | 1 | Bone fracture | + | + | + | Hypotonic | − | + | + | + | + | − | − | Born with teeth | 9 |
| 2 | F | − | − | 1 | Incidental | + | + | − | Normal | − | + | + | + | + | + | + | Gum hypertrophy | 9 |
| 3 | F | − | + | 1 | Hematemesis | + | + | − | Normal | + | + | + | + | − | − | + | Corpus callosum lipoma | Lost to follow-up |
| 4 | F | + | + | 1.5 | Incidental | + | + | + | Axial hypotonia and limbs hypotonia | + | + | + | + | + | − | − | Multiple styles | Lost to follow-up |
| 5 | M | + | + | 1 | Incidental | + | + | + | Hypertonic | − | + | + | + | + | + | − | Bilateral hydrocele and left inguinal hernia | 11 |
| 6 | F | + | + | 2 | Incidental | + | + | + | Hypertonic | − | + | + | + | + | − | + | − | 13 |
| 7 | M | + | + | 2 | Incidental | + | + | + | Hypertonic | + | + | + | + | + | − | + | Bilateral hydrocele | Lost to follow-up |
| 8 | F | − | + | 1.5 | Paleness | − | + | + | Normal | − | + | + | + | − | − | − | − | 10 |
| 9 | M | − | + | 1.5 | Paleness and irritability | + | − | + | Hypotonic | + | + | + | + | − | − | − | Left pelvic kidney | 10 |
| 10 | M | − | + | 1.5 | Incidental | + | + | + | Axial hypotonia and limbs hypotonia | + | + | + | + | − | − | + | Membranous cleft palate | 12 |
| 11 | M | + | + | 1 | Incidental | + | + | + | Normal | − | + | + | + | − | + | + | Bilateral inguinal hernia | 2 |
| 12 | M | − | + | 1.5 | Paleness | + | + | − | Hypertonic | + | + | + | + | − | − | − | − | 14 |
| 13 | M | − | + | First week | Screening | + | + | + | Normal | − | + | + | + | − | + | − | − | 7 |
| 14* | F | + | + | First week | Screening | + | + | + | Normal | − | + | + | + | − | + | − | Gum hypertrophy and umbilical hernia | 14 |
| 15 | F | + | + | First week | Screening | + | + | + | Normal | − | + | + | + | − | + | − | − | 10 |
| 16 | F | − | + | 4 | Paleness and failure to thrive | + | + | + | Axial hypotonia and limbs hypotonia | + | + | + | + | − | − | − | − | 18 |
| 17 | F | + | + | 1.5 | Incidental | + | + | + | Normal | − | + | + | + | − | + | + | − | 12 |
| 18 | F | + | + | 1 | Screening | + | + | − | Normal | + | + | + | + | − | − | − | − | 11 |
| 19 | F | − | + | 4 | Failure to thrive and developmental delay | + | + | + | Dystonic | − | + | + | + | − | − | − | − | 14 |
| 20 | M | − | + | 2 | Poor activity, poor sucking, petechial rash | + | + | + | Normal | − | + | + | + | − | − | + | − | 12 |
| 21 | F | − | + | 3 | Reduced feeding and irritability | + | + | + | Normal | + | + | + | + | − | + | − | − | 6 |
| 22* | M | + | + | First week | Screening | + | + | + | Hypertonic | − | + | + | + | − | − | − | Bilateral inguinal hernia | 13 |
- Note: Ages in numbers are in months. + = positive clinical finding. − = history or clinical finding not present. * Offspring of the same couple. ** Diagnosis made clinically that warranted genetic testing.
2.1 Family and obstetric history
Consanguinity was reported in all parents except those of Patient 2. 45.5% of patients had either a sibling or cousin diagnosed with OSTM1 MIOP. Patients 1, 14, and 22 were born to the same couple. Eighty-six percent of patients had the same tribal family name.
Ninety-five percent of patients had an uneventful prenatal history and were born at term with a normal birth weight of ~3 kg. Patient 2 was 33 weeks premature, with a birth weight of 1.8 kg. All infants gained ~1 kg in the first month of life but, after that, 95% failed to gain weight and maintained the same birth weight plus 1 kg throughout their lives, irrespective of increasing calorie intake through feeds or high-calorie formula feeds. Only Patient 8 gained ~3 kg, but even her weight was at the 10th centile by 6 months of age, and her weight dropped two centiles on the growth chart over those 6 months.
2.2 Presenting features and diagnosis
A majority of patients (72.7%) were diagnosed before 2 months of age and all by 4 months. The most common reason for diagnosis was an incidental presentation and high clinical suspicion. Eight patients presented to their primary doctor or the emergency room with unrelated symptoms and signs such as jaundice, a common cold, or fever, although Patient 10 presented with sepsis. All eight patients presenting in this manner had hepatosplenomegaly on clinical examination, increased bone density through imaging, and pancytopenia through blood investigations warranting further investigation and specialist referral before confirming the MIOP diagnosis through genetic testing.
Five patients were tested directly for OSTM1 due to the family history. The third most common presenting complaint (in four patients) was paleness due to anemia.
2.3 Symptoms common to all patients
All 22 patients developed upper respiratory symptoms, hepatosplenomegaly, poor feeding, and had severe developmental delay. The upper respiratory symptoms, presenting at 2–3 months of age, included noisy breathing, tachypnea, and sometimes reduced oxygen saturation, and these features were often misdiagnosed as a respiratory infection such as acute bronchiolitis. By 6 months, these symptoms become severe and required recurrent suctioning. By 9–12 months, many patients required home oxygen and, at the end of life, some patients required continuous non-invasive ventilation.
All patients had hepatosplenomegaly, including the patients who were diagnosed in the first week of life.
Difficulty in feeding started at different ages (range 2 weeks to 11 months). However, most were on tube feeding by 6 months and, at the end of life, many of the patients had difficulty swallowing their saliva.
No patient progressed normally through their developmental milestones during their lives.
2.4 Neurological complications
Over 80% of patients developed pain, irritability, or both to various degrees. For example, Patient 21 had such severe pain and irritability that it warranted extensive investigations to look for a cause. Patients 1, 8, 10, 11, and 14 required benzodiazepines or oral sedatives to manage irritability or constant crying. Many patients were reported to cry on gentle touching. Despite reported pain or irritability in many patients, we speculate that these features were still underreported. Symptoms were diagnosed at different ages and were not associated with disease progression.
The pain and/or irritability was evident in the daily management of the children. While most patients did not receive a systematic pain evaluation, descriptors of pain behaviors were conspicuous in the clinical and nursing notes. For example, six patients were not reported to have irritability nor pain on admission, but their symptoms, signs, or management reflected that they were experiencing these symptoms. Five of these patients were prescribed drugs for “irritability” as needed, and indeed received several doses, and one patient had written instructions to “bundle gently to prevent pain”. Clinical symptoms and signs that were likely to have reflected pain behavior included Patient 3, who had “unexplained tachycardia”; Patients 4, 10, and 16, who were described as “stiff hypotonic”; and Patient 19 who was diagnosed with “dystonic movements.” This observation was not limited to our hospital. Patient 5 was referred to a tertiary center in the United States for possible HCT. At 9 months, he was referred to a psychiatrist to manage “delirium.” The psychiatrist diagnosed it as pain behavior and recommended opioids.
The muscle tone of the patients was associated with the disease stage and may have indicated relentless progressive neurological deterioration. Early in the disease course around diagnosis, the tone was usually normal or axial hypotonia. After 6 months, patients became more hypotonic with hyperreflexia. However, their pain behavior may have affected the accuracy of hypertonicity. For example, Patient 21 was diagnosed with limb hypertonia which improved when she received proper pain management and regained spontaneous movements (Alotaibi & Dighe, 2021).
Seizures without any secondary cause (e.g., hypocalcemia) were diagnosed in 40.9% of patients. Seizure onset was variable from 2 to 18 months. Seizures were characterized by a cluster of flexor tonic spasms that lasted for up to 20 min, similar to infantile spasms. Two other patients had secondary causes for their seizures (intracranial bleeding in the first week of life and at 2 months), but their seizures were clinically distinct tonic–clonic seizures.
Hearing assessments (brainstem auditory evoked potential tests) were recorded in only three patients at 2 weeks, 1 month, and 2 months, which were normal. The ophthalmological records were also incomplete; however, three patients had normal visual evoked potential examinations at 1 week and 6 weeks. Patient 5 had a normal visual evoked potential examination at 1 month but became blind at 9 months, and the ophthalmological examination showed bilateral optic atrophy. Five patients were reported to lack fixation at 2 months of age.
2.5 Musculoskeletal features
Bone fractures were unexpectedly rare, as only 27% of patients developed fractures. Only Patient 1 had a fracture at birth. The other patients who developed fractures tended to have had more laboratory investigations and their sites of fracture were usually the limbs that had blood draws. Hence, we speculate that the other fractures were iatrogenic.
Eight patients developed spontaneous painful soft tissue swellings in one of their limbs. Clinically, these did not resemble dermal inflammation and radiological investigations ruled out fractures. All swellings followed a history of blood draws. The location was not the injection site but at the manipulation site to take blood by a health professional. The swellings were self-limiting and resolved in a few days.
2.6 Hematological features
In most patients, bleeding caused by thrombocytopenia was mainly seen as epistaxis after aggressive suctioning. Only Patient 3 had spontaneous bleeding in the form of hematemesis. Patients 1 and 20 had Intraventricular hemorrhage.
2.7 Other features
Other findings seen in at least two patients throughout disease course included gum hypertrophy and hernias.
2.8 Mortality and hematopoietic cell transplantation
The mean age of death was 10.9 months, not including patients lost to follow-up (range 2–18 months). One patient underwent HCT, which failed.
2.9 Laboratory and radiological investigations
On presentation, microcytic or normocytic anemia (hemoglobin between 36 and 96 g/L, normal range 140–200 g/L) and thrombocytopenia (platelet count <60,000/mm3, normal range 150,000–450,000/mm3) were present in all patients except for Patient 10, whose platelet count was 143,000/mm3. White blood cells were either normal or immature cells were increased.
A blood film was performed in 12 patients, which confirmed the complete blood count. However, other distinctive findings were seen on most blood films (leukoerythroblastosis), and >80% of blood films showed monocytosis.
Abdominal ultrasound (US) confirmed hepatosplenomegaly in all patients and increased bone density was present in plain X-rays early in the course of the disease.
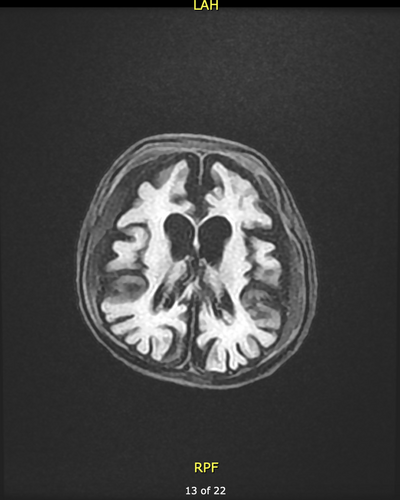
Half of the patients had one or more brain imaging procedures (US, CT, or MRI). In patients with imaging performed at 7 months or earlier, the brain structure was normal. However, imaging after 7 months revealed demyelination and brain atrophy (Figure 1).
Patients 16 and 20 had CT scanning of the paranasal sinuses as part of their investigations for upper airway symptoms at 7.5 months and 1 year, respectively. Both revealed non-pneumatization of all paranasal sinuses.
For patients with primary seizures, their electroencephalograms reported poor organized symmetrical low amplitude frequencies (3–4 Hz) suggestive of severe generalized encephalopathic changes.
3 DISCUSSION
OSTM1 mutations are responsible for <5% of cases of MIOP (Askmyr et al., 2008; Balemans et al., 2005; Liu et al., 2021; Mazzolari et al., 2009; Pangrazio et al., 2006; Penna et al., 2019; Sobacchi et al., 2013; Villa et al., 2009). OSTM1 is located on chromosome 6q21, and the mutation was described first by Ramírez et al. in humans in 2004 (Ramirez et al., 2004). Our review of the literature found only 22 patients with confirmed OSTM1 MIOP in 15 publications, mainly from Kuwait, Lebanon, Saudi Arabia, India, Italy, and Pakistan, and another three patients were described as Arabs. Consanguinity was seen in most parents of affected individuals (Abinun et al., 1999; Adel et al., 2013; Alroy et al., 2007; Askmyr et al., 2008; Bubshait et al., 2020; Chiodo et al., 2007; Heraud et al., 2014; Herebian et al., 2017; Maranda et al., 2008; Overholt et al., 2017; Pangrazio et al., 2006; Quarello et al., 2004; Ramirez et al., 2004; Sobacchi et al., 2013; Souraty et al., 2007; Strauss et al., 2015). The clinical features of published 22 patients with OSTM1 MIOP are summarized in Table 2.
| Publication | Gender | Nationality/ethnicity | Consa-nguinity | Brain atrophy or microcephaly | Muscle tone | Developmental delay | Pain or irritability | Failure to thrive | Vision abnormalities | Hearing abnormalities | Seizures | Upper airway difficulties | Feeding difficulties | Hepatosplenomegaly | Fractures | Significant bleeding | Gum hypertrophy | Age at death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ramirez et al. (2004) | M | Kuwait | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A | − | N/A | N/A | N/A | − | − | N/A | 6 |
| F | Kuwait | + | N/A | Hypertonia | N/A | N/A | N/A | + | N/A | − | N/A | N/A | + | − | − | N/A | N/A | |
| Pangrazio et al. (2006) | F | Kuwait | + | + | Hypertonia | N/A | N/A | + | + | − | − | N/A | N/A | + | − | − | + | N/A |
| Souraty et al. (2007) | M | Lebanon | + | + | Hypotonia | + | N/A | + | + | N/A | + | N/A | + | − | − | − | N/A | 9 |
| M | Lebanon | + | + | N/A | N/A | N/A | + | N/A | N/A | − | N/A | N/A | + | + | + | N/A | 3.5 | |
| Chiodo et al. (2007) | F | Kuwait | + | + | limb hypertonia, axial hypotonia | N/A | + | + | + | N/A | − | N/A | N/A | + | − | − | + | N/A |
| Maranda et al. (2008) | F | N/A | + | + | limb hypertonia, axial hypotonia | + | + | + | + | + | + | N/A | + | + | + | + | + | 12 |
| Mazzolari et al. (2009) | N/A | N/A | N/A | + | Hypotonia | + | N/A | + | + | N/A | + | + | N/A | + | − | − | N/A | 19 |
| N/A | N/A | N/A | − | Hypotonia | + | N/A | + | + | N/A | + | + | N/A | + | + | − | N/A | 11 | |
| N/A | N/A | N/A | + | Hypotonia | + | N/A | + | + | N/A | + | + | N/A | + | + | − | N/A | 31 | |
| Ott et al. (2013) | M | Arab | + | + | N/A | N/A | N/A | N/A | N/A | N/A | + | N/A | N/A | + | − | − | N/A | 1.5 |
| M | Arab | + | N/A | N/A | N/A | N/A | N/A | + | + | − | N/A | N/A | N/A | − | − | N/A | 4 | |
| F | India | + | + | N/A | + | N/A | N/A | + | + | + | N/A | N/A | + | − | − | + | 5 | |
| Adel et al. (2013) | N/A | Arab | + | + | limb hypertonia, axial hypotonia | + | + | + | + | N/A | + | N/A | − | − | − | − | N/A | N/A |
| Strauss et al. (2015) | M | N/A | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | + | N/A | − | N/A | N/A |
| Overholt et al. (2017) | M | N/A | − | + | N/A | + | N/A | + | + | N/A | + | + | + | N/A | − | − | N/A | N/A |
| Herebian et al. (2017) | M | Saudi Arabia | + | + | Hypertonia | + | + | N/A | + | N/A | + | N/A | + | N/A | − | − | N/A | N/A |
| Bubshait et al. (2020) | F | Saudi Arabia | + | − | limb hypertonia, axial hypotonia | + | N/A | + | + | N/A | + | + | N/A | + | − | − | N/A | 6 |
| Liu et al. (2021) | M | Pakistan | + | − | N/A | + | N/A | N/A | − | − | − | N/A | N/A | + | − | − | N/A | N/A |
| Abinun et al. (1999) and Pangrazio et al. (2006) | M | Kuwait | + | + | Hypertonia | N/A | N/A | N/A | + | − | − | N/A | N/A | N/A | + | − | N/A | N/A |
| Quarello et al. (2004) and Pangrazio et al. (2006) | M | Italy | − | + | Hypotonia | N/A | N/A | + | N/A | N/A | + | N/A | N/A | + | − | − | N/A | 1 |
| Pangrazio et al. (2006), Souraty et al. (2007), and Alroy et al. (2007) | M | Lebanon | + | − | Hypotonia | N/A | N/A | + | + | N/A | − | N/A | N/A | + | + | + | N/A | 5 |
- Note: Ages in numbers are in months. + = positive clinical finding. − = history or clinical finding not present. N/A = not available.
OSTM1 mutation causes a multi-systemic disease, as the gene is expressed in various tissues including the brain, heart, liver, kidney, and bone (Chalhoub et al., 2003). However, the exact repertoire of OSTM1 functions remains unknown (Ott et al., 2013; Shin et al., 2014; Vacher et al., 2020). The best characterized function is the formation of the OSTM1 beta-subunit and the support of the CLC-7 channel in lysosomes on osteoclasts, which, if absent, interferes with osteoclast secretory function (Lange et al., 2006; Leisle et al., 2011; Mazzolari et al., 2009; Pressey et al., 2010). However, OSTM1 defects are also responsible for neurodegeneration, osteoclastogenesis dysfunction, and interrupted hematopoietic lineages (Barrallo-Gimeno et al., 2015; Heraud et al., 2014; Ott et al., 2013; Pata et al., 2008; Pressey et al., 2010; Shin et al., 2014; Sobacchi et al., 2013; Vacher et al., 2020; Weinert et al., 2014). We therefore group comorbidities into these systemic classifications.
3.1 Neurological complications
According to mouse studies, high OSTM1 expression in nerve cells causes staged neurological degeneration. The first stage is gliosis with an increase in astrocytes and microglia. In a second stage, myelination defects, massive neurodegeneration, and brain atrophy develop (Ott et al., 2013; Prinetti et al., 2009; Shin et al., 2014; Vacher et al., 2020). The overall pathological features resemble neurodegenerative lysosomal storage disorders (Alroy et al., 2007; Barrallo-Gimeno et al., 2015; Chiodo et al., 2007; Herebian et al., 2017; Prinetti et al., 2009; Shin et al., 2014; Weinert et al., 2014). These phased changes are likely to underpin the imaging heterogeneity seen in patients and perhaps the variability in muscle tone. Indeed, later imaging in our patients revealed global brain changes and atrophy, and the literature describes both normal brain imaging in early disease and atrophy in later disease (Abinun et al., 1999; Adel et al., 2013; Alroy et al., 2007; Bubshait et al., 2020; Chiodo et al., 2007; Herebian et al., 2017; Maranda et al., 2008; Ott et al., 2013; Overholt et al., 2017; Pangrazio et al., 2006; Quarello et al., 2004; Ramirez et al., 2004; Souraty et al., 2007; Strauss et al., 2015). In reported studies, muscle tone ranged from hypotonic, axial hypotonia with limb hypertonia to hypertonia with increased reflexes (Abinun et al., 1999; Adel et al., 2013; Alroy et al., 2007; Bubshait et al., 2020; Chiodo et al., 2007; Herebian et al., 2017; Maranda et al., 2008; Mazzolari et al., 2009; Pangrazio et al., 2006; Quarello et al., 2004; Ramirez et al., 2004; Souraty et al., 2007). However, it is difficult to rule out pain as an underlying cause of increased muscle tone. Indeed, Hauer et al. described that patients with significant neurological impairments develop characteristic behavioral changes to express pain including “stiffens or spasms,” “spastic,” “tense,” “rigid,” and “marked increase in spasticity,” which clinicians may mistake as hypertonia rather than pain (Hauer et al., 2017).
Pain and irritability were very severe in our cases and some of the cases reported in the literature (Adel et al., 2013; Chiodo et al., 2007; Herebian et al., 2017; Maranda et al., 2008). However, this phenomenon was not comprehensively documented and was even misdiagnosed as other causes in the medical records. This is not uncommon as, in general, pain in children is often inadequately assessed, misdiagnosed, and mistreated (CoPAo & Health, 2001; Hauer et al., 2017; Jain et al., 2012; Mathews, 2011; Zernikow et al., 2012). Furthermore, due to the generalized neurological findings seen in all of these patients, it is likely that pain, irritability, or both are present in all cases. Regarding the source of pain, it is unlikely that the pain is of bony origin, as similar pain is absent in other forms of osteopetrosis (Mazzolari et al., 2009; Sobacchi et al., 2013; Villa et al., 2009). The distinctive feature of OSTM1 MIOP is the extensive nervous system involvement. Therefore, we speculate that nervous system disease causes the pain. Patients with neurological sequelae have been described as having “neurological irritability” or “central neuropathic pain.”(Hauer et al., 2017; Rasmussen & Gregoire, 2015; Walker, 2020) However, in 2017, the International Association for the Study of Pain added nociplastic pain as a third type of pain, a designation consistent with our patients' symptoms. Nociplastic pain is defined as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain” (International Association for the Study of Pain [IASP], 2022). This type of pain is caused by central sensitization through nervous system dysfunction (IASP, 2022; Latremoliere & Woolf, 2009; Nijs et al., 2021). Nociplastic pain is associated with hyperalgesia, allodynia, and other comorbidities like fatigue or sleep disturbance (IASP, 2022; Nijs et al., 2021). Many of our patients had allodynia, hyperalgesia, irritability, or disturbed sleep, entirely consistent with this diagnosis. There are treatment implications of the fact that pain in children with OSTM1 osteopetrosis is predominantly nociplastic. Nociplastic pain might respond to a combination of analgesics like opioids and adjuvant analgesics like gabapentinoids rather than analgesics alone (Fitzcharles et al., 2021; Hauer et al., 2017; Nijs et al., 2021).
Based on the observations from our case series, we believe that routine proactive assessment of pain and early management with appropriate medical and non-pharmacological treatments should be an essential part of care for these children.
Our reported incidence of seizures was remarkably similar to other published cases, occurring in 12 out of 22 published cases compared with 11 (9 primary and 2 secondary seizures) out of 22 patients in our study, which, when described, mostly resembled infantile spasms (Bubshait et al., 2020; Heraud et al., 2014; Herebian et al., 2017; Maranda et al., 2008; Overholt et al., 2017; Ramirez et al., 2004; Strauss et al., 2015).
In the literature, and consistent with our series, developmental delay was described as severe in many patients with hardly any progression of development (Adel et al., 2013; Bubshait et al., 2020; Herebian et al., 2017; Mazzolari et al., 2009; Ott et al., 2013; Overholt et al., 2017; Quarello et al., 2004; Souraty et al., 2007). Blindness and deafness were other common symptoms, as described by other case studies. Visual defects were reported more than auditory, and they tend to develop over time, with patients normal at early presentation followed by blindness in advanced disease (Abinun et al., 1999; Adel et al., 2013; Bubshait et al., 2020; Chiodo et al., 2007; Herebian et al., 2017; Maranda et al., 2008; Mazzolari et al., 2009; Ott et al., 2013; Overholt et al., 2017; Pangrazio et al., 2006; Ramirez et al., 2004; Souraty et al., 2007; Vacher et al., 2020). Ophthalmological examination of affected children has revealed retinal dystrophy with pale optic discs (Chiodo et al., 2007; Maranda et al., 2008; Overholt et al., 2017; Pangrazio et al., 2006). The literature describes that neuropathic MIOPs, including OSTM1, are characterized by retinal atrophy and sensorineural deafness caused by neurodegeneration (Abinun et al., 1999; Barrallo-Gimeno et al., 2015; Heraud et al., 2014; Lange et al., 2006; Maranda et al., 2008; Ott et al., 2013; Overholt et al., 2017; Pangrazio et al., 2006; Pressey et al., 2010; Sobacchi et al., 2013; Stark & Savarirayan, 2009). We speculate that the visual and hearing defects in our presented cases are sequelae of primary neurodegeneration similar to what was found in the literature (Ott et al., 2013; Vacher et al., 2020; Villa et al., 2009; Wu et al., 2017). This is suggested by not identifying significant narrowing of skull foramina on images, the absence of concurrent clinical findings suggestive of nerve obstruction like cranial nerves, and the presence of brain atrophy.
It is plausible that feeding difficulties arise due to neurodegeneration of the swallowing apparatus, unlike our previous hypothesis that it is caused by an inability to breathe (Alotaibi & Dighe, 2021). In most patients reported in the literature, feeding difficulties present early (Herebian et al., 2017; Maranda et al., 2008; Overholt et al., 2017; Souraty et al., 2007).
3.2 Orthopedic complications
The bone complications are not unlike those seen in other types of MIOP, although arguably more severe in OSTM1 MIOP (Balemans et al., 2005; Mazzolari et al., 2009; Sobacchi et al., 2013; Stark & Savarirayan, 2009; Villa et al., 2009). The upper respiratory tract is affected by narrowing and distortion of the nasal architecture with poorly pneumatized sinuses. Narrowing varies in severity at birth from not detectable to choanal stenosis and atresia (Al-Mofada et al., 1993; Fitzcharles et al., 2021; Mazzolari et al., 2009; Villa et al., 2009; Wu et al., 2017). The symptoms are progressive, and most patients eventually develop obstructive sleep apnea or need positive pressure ventilation (Al-Mofada et al., 1993; Kasow et al., 2008; di Palmo et al, 2018).
Although fractures were uncommon in our patients, fractures appear to be more common in OSTM1 MIOP patients than in other types of MIOP reported in the literature (Mazzolari et al., 2009). However, macrocephaly was absent in reported OSTM1 MIOP cases, as seen in our patients, with the exception of one case reported by Liu et al. (Abinun et al., 1999; Adel et al., 2013; Ott et al., 2013; Pangrazio et al., 2006; Sobacchi et al., 2013; Souraty et al., 2007).
Soft tissue swellings possibly related to blood draws or IV cannulation were seen in eight of our patients. We advocate that children with OSTM1 MIOP are only subjected to invasive procedures, intravenous antibiotics, and blood transfusions with due consideration of the risks versus the benefits. Most of our patients had preemptive blood draws that did not significantly change their management. For example, Patient 2 had complete blood tests performed almost every day for 1 week during one of her admissions.
3.3 Hematological complications
Expansion of bone into the bone marrow space in patients with OSTM1 mutations, like in other types of osteopetrosis, results in a failure of normal hematopoiesis that causes transfusion-dependent anemia and thrombocytopenia. In addition, extramedullary hematopoiesis results in early hepatosplenomegaly (Bubshait et al., 2020; Chiodo et al., 2007; Liu et al., 2021; Maranda et al., 2008; Mazzolari et al., 2009; Ott et al., 2013; Pangrazio et al., 2006; Ramirez et al., 2004; Souraty et al., 2007; Strauss et al., 2015; Villa et al., 2009; Wu et al., 2017). Most bleeding episodes were minor in our cases. However, three cases in the literature developed significant bleeding episodes due to thrombocytopenia (Maranda et al., 2008; Pangrazio et al., 2006; Souraty et al., 2007).
In a study of Ostm1 mutations in mice, OSTM1 plays a role in several hematopoietic cell types and consequently its mutation deregulates multiple hematopoietic lineages resulting in high B, NK, and mast cell counts and lower T cell and macrophage counts (Pata et al., 2008). Furthermore, due to the aplastic anemia and blood film findings, several reported cases have prompted a diagnosis of juvenile myelomonocytic leukemia (Hoyoux et al., 2014; Jain et al., 2017; Strauss et al., 2015).
3.4 Other
Severe growth failure is commonly reported in patients with OSTM1 mutation, similar to our cases (Adel et al., 2013; Bubshait et al., 2020; Chiodo et al., 2007; Herebian et al., 2017; Maranda et al., 2008; Overholt et al., 2017; Pangrazio et al., 2006; Sobacchi et al., 2013; Souraty et al., 2007). On the other hand, gum hypertrophy was only described in four cases in the literature and two of our cases (Chiodo et al., 2007; Maranda et al., 2008; Ott et al., 2013; Pangrazio et al., 2006). The consistency of the gums was not described very well in our reported cases nor in the literature. However, Sartelet et al. described large gingival hamartomas in large animals with OSTM1 mutations (Sartelet et al., 2014).
3.5 Prognosis
Most cases reported in the literature did not survive beyond the age of 2, similar to our patients, and the leading cause of death was severe neurologic deterioration (Mazzolari et al., 2009; Ott et al., 2013; Overholt et al., 2017; Pangrazio et al., 2006; Souraty et al., 2007).
HCT is the only curative treatment for MIOP, but it is usually not offered to patients with OSTM1-related disease due to their severe neurological deficits (Chiodo et al., 2007; Herebian et al., 2017; Maranda et al., 2008; Mazzolari et al., 2009; Ott et al., 2013; Pangrazio et al., 2006). Nevertheless, two patients have received successful transplants. Although both patients survived beyond the age of 2, both continued to develop significant neurological complications (Herebian et al., 2017; Overholt et al., 2017).
4 STUDY LIMITATIONS
There are some limitations to this study. First, there might be population bias, as most patients in the case series belonged to one family tribe. Hence, the observed phenotype might reflect that specific genetic pool. Second, the medical documentation was generally poor, incomplete, and paper-based, so there may be missing or incomplete data. Third, three patients were lost to follow-up, and despite our best efforts, we were unable to connect with these patients' families. Fourth, the patient population include only cases from a single center. Finally, the sample size was very small due to the rarity of this disease.
5 CONCLUSION
OSTM1 is expressed in various tissues including the brain, heart, liver, kidney, and bone, resulting in multi-system dysfunction, especially severe neurodegeneration resembling neurodegenerative lysosomal storage disorders. As a result, OSTM1 MIOP is a systemic disease with a pathogenesis that extends beyond osteoclast failure alone. Hence, we recommend that OSTM1 MIOP is classified as a “syndrome” that includes osteopetrosis as a comorbidity. We also recommend early palliative care referral due to the disease's lethality in early life and symptom severity, especially with respect to pain and irritability.
FUNDING INFORMATION
The authors have solely funded this work.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




