Expanding SPG7 dominant optic atrophy phenotype: Infantile nystagmus and optic atrophy without spastic paraplegia
Jinu Han and Byung Joo Lee equally contributed to this work as joint corresponding authors.
Funding information: Korea Centers for Disease Control and Prevention, Grant/Award Numbers: 2018-ER6902-02, 2019-NG-051-01; National Research Foundation of Korea; Korea Government, Grant/Award Number: 2020R1C1C1007965
Abstract
Spastic paraplegia is a neurodegenerative disorder characterized by progressive leg weakness and spasticity due to degeneration of corticospinal axons. SPG7 encodes paraplegin, and pathogenic variants in the gene cause hereditary spastic paraplegia as an autosomal recessive trait. Various ophthalmological findings including optic atrophy, ophthalmoplegia, or nystagmus have been reported in patients with spastic paraplegia type 7. We report a 15-year-old male patient with a novel heterozygous variant, c.1224T>G:p.(Asp408Glu) in SPG7 (NM_003119.3) causing early onset isolated optic atrophy and infantile nystagmus prior to the onset of neurological symptoms. Therefore, SPG7 should be considered a cause of infantile nystagmus with optic atrophy.
1 INTRODUCTION
SPG7 encodes paraplegin that is located at the mitochondrial inner membrane and composed of three domains: (1) N-terminal FtsH-extracellular domain; (2) intermediate AAA domain (ATPase associated with diverse cellular activities), and (3) C-terminal metallopeptidase (Karlberg et al., 2009). The mitochondrial AAA protease is an ATP-dependent proteolytic complex that degrades misfolded protein and regulates ribosome assembly. Pathogenic variants in this gene are known to cause hereditary spastic paraplegia as an autosomal recessive trait (Elleuch et al., 2006). Spastic paraplegia type 7 (OMIM #607259) is characterized by progressive spastic paraparesis, hypertonic bladder, and sensory dysfunction of lower limbs (Eriksen et al., 2022). Most of the variants reported in spastic paraplegia are loss-of-function variants, sometimes it can develop axonal-sensorimotor polyneuropathy or subcortical dementia (Klebe et al., 2012). Varying degrees of optic atrophy have been identified in nearly all patients (Klebe et al., 2012), but other studies reported that optic atrophy was seen only in 1 patient among 42 cases (Hewamadduma et al., 2018). Other ophthalmological findings, such as ophthalmoplegia, nystagmus, strabismus, and blepharoptosis have also been reported (Eriksen et al., 2022; Pfeffer et al., 2014). Although most patients with spastic paraplegia had two pathogenic variants in SPG7, dominant negative effect has been suggested in patients carrying a single heterozygous p.(Leu78*) variant (Sánchez-Ferrero et al., 2013). Recently, a single heterozygous variant in SPG7 has been suggested as a cause of isolated optic atrophy without neurologic phenotype (Charif et al., 2020; Charif et al., 2021). Herein, we describe a novel heterozygous SPG7 missense variant causing infantile-onset jerk nystagmus and optic atrophy without neurologic symptoms.
2 MATERIALS AND METHODS
2.1 Case report
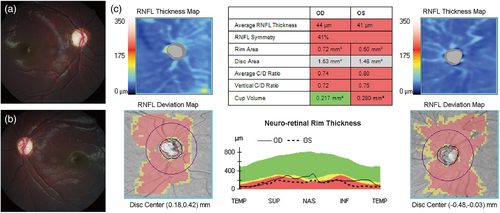
A 4-month-old boy presented with 2–3 Hz horizontal pendular nystagmus and optic atrophy. He was born after an uneventful pregnancy. There was no family history of visual impairments or neurological disorders. On flash visual-evoked potentials, he showed markedly prolonged P100 latencies in both eyes. He underwent strabismus surgery for V-pattern exotropia and bilateral dissociated vertical deviations when he was 5 years old. No structural abnormalities were noted on brain magnetic resonance imaging. There were no delayed development and intellectual disability. Referred to pediatric neurology consultation for evaluating systemic abnormalities, neurologic examination showed normal muscle tone and strength, normal gait, and no cognitive impairment. At the age of 15, he denied any walking difficulties or leg stiffness. The best corrected visual acuity was 20/40 in the right eye and 20/60 in the left eye. Slit-lamp examination results were unremarkable. Dilated fundus examination revealed small optic disc and optic disc pallor in both eyes (Figure 1a,b). Diffuse thinning of the peripapillary retinal nerve fiber layer (RNFL) was noted on optical coherence tomography (OCT) (Figure 1c). Ocular motility examination revealed 2–3 Hz left beating jerk nystagmus (Video S1).
3 RESULTS
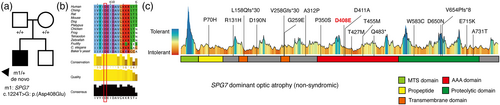
Exome sequencing (Twist Comprehensive Exome; Twist Bioscience, San Francisco, CA, USA) was performed. Informed written consent was obtained. This study was approved by the Institutional Review Board of Gangnam Severance Hospital (3-2020-0063) and adhered to the tenets of the Declaration of Helsinki. A novel heterozygous c.1224T>G:p.(Asp408Glu) variant was identified in SPG7 (NM_003119.3) (Figure 2a). This variant was absent in gnomAD, TOPMed, and the Korean Reference Genome Database. It is located in the functional AAA domain and is predicted to be deleterious in multiple in silico prediction tools (CADD: 22.2, SIFT: 0, PolyPhen-2: 1, FATHMM: 0.645) (Figure 2b). There were no copy number variations in the known optic atrophy gene, as assessed using ExomeDepth (Plagnol et al., 2012). Subsequently, a trio exome analysis revealed that it occurred de novo. This variant was classified as likely pathogenic, according to the American College of Medical Genetics guidelines: PS2, de novo (both maternity and paternity confirmed) in a patient with the disease and no family history; PM1, located in a mutational hot spot and/or critical and well-established functional domain without benign variation; PM2, absent from controls (or at extremely low frequency if recessive) in Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium (Richards et al., 2015).
4 DISCUSSION
SPG7 gene is located in 16q24.3, and it encodes 795 amino acids paraplegin protein. The protein forms the hetero-oligomeric protease complexes with the homologous ATPase AFG3L2, which act as a mitochondrial inner membrane metalloprotease (m-AAA protease). It is involved in the cleavage of OPA1 (Pfeffer et al., 2014), which is crucial in maintaining mitochondrial function by promoting inner membrane fusion of mitochondria and regulating mitochondrial cristae morphology. Moreover, this protein complex is required for proper mitochondrial permeability transition pore opening (Sambri et al., 2020), which is essential to mitochondrial calcium homeostasis. Although SPG7 behaves predominantly as a recessive gene for hereditary spastic paraplegia, it can also act as a dominant gene for isolated optic atrophy (Charif et al., 2020; Charif et al., 2021). To date, 17 heterozygous SPG7 variants have been reported to cause isolated dominant optic atrophy (Figure 2c) (Charif et al., 2020; Charif et al., 2021; Klebe et al., 2012). Among them, 14 were missense variants and 4 were loss-of-function variants. Visual acuity in SPG7 dominant optic atrophy varied from 20/200 to 20/20. Previous studies have reported that visual impairment occurred during 30–60 years of age in most cases and RNFL thinning was noted mainly in the temporal sector (Charif et al., 2020). Unlike previous cases, our patient had infantile nystagmus and optic atrophy since early infancy and severe generalized RNFL thinning was noted on OCT. Despite of early-onset severe RNFL thinning, visual acuity was consistent with previous reports. Although pendular nystagmus or gaze-evoked nystagmus with early-onset optic atrophy had been reported in patients with homozygous or compound heterozygous SPG7 variants (Eriksen et al., 2022; Marcotulli et al., 2014), our case is unique because a heterozygous SPG7 missense variant causes infantile-onset jerk nystagmus with optic atrophy.
Although we did not exclude the possibility of hidden non-coding or deep intronic variant in this patient, the presumed causative c.1224T>G:p.(Asp408Glu) heterozygous variant is located in the AAA domain, which is a highly conserved region. The variant glutamate residues are also located in the proximity of the nucleotide-binding cleft, which might affect the stability of the protein and ATP-binding site (Karlberg et al., 2009). Since all reported SPG7 missense variants in dominant optic atrophy were interspersed throughout four functioning domains and non-functional domains, it might not be associated with specific functional domain. Since paraplegin interacts with AFG3L2 to form hetero-oligomer, the mutant protein may interfere with the formation of hetero-oligomer. In addition, the mechanism of haploinsufficiency may also play a role in causing optic atrophy because null variants were also reported in SPG7 dominant optic atrophy. However, further validation study is needed to verify why certain missense variants are inherited as dominant and cause optic atrophy without neurological phenotype.
Our patient did not show any neurological signs or symptoms until the age of 15 years. Ophthalmological manifestations, including infantile nystagmus, optic atrophy, and reduced visual acuity, are the sole clinical manifestations of SPG7-dominant optic atrophy. A previous study reported that spasticity or ataxia occurred at a mean age of 35 years in patients with biallelic SPG7 variants (Coarelli et al., 2019). Despite the absence of neurological phenotypes at present, regular neurological assessments should be performed as neurological symptoms may develop when he is in middle age. In addition, further study is needed to investigate the natural history and annual rate of RNFL thinning in patients with SPG7 dominant optic atrophy.
Here, we report a novel heterozygous c.1224T>G:p.(Asp408Glu) SPG7 variant in a patient with early-onset optic atrophy and infantile nystagmus, without spastic paraplegia. Although typical onset of spastic paraplegia and optic atrophy caused by SPG7 variants is mostly in adulthood, some variants might lead to early mitochondrial dysfunction (Charif et al., 2020). We propose that SPG7 should be considered a causative gene for infantile nystagmus with optic atrophy.
AUTHOR CONTRIBUTIONS
Yuri Seo and Jinu Han have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. Yuri Seo, Byung Joo Lee, and Jinu Han were involved in drafting the manuscript or revising it critically for important intellectual content. All authors were involved in reviewing and editing the manuscript.
FUNDING INFORMATION
This study was supported by the Korea Disease Control and Prevention Agency (Grant Nos. 2018-ER6902-02 and 2019-NG-051-01) and a National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2020R1C1C1007965).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




