Clinical phenotype and musculoskeletal characteristics of patients with aggrecan deficiency
Eirene Alexandrou and Andrew Dauber co-first author.
Abstract
Aggrecan is a proteoglycan within the physeal and articular cartilage. Aggrecan deficiency, due to heterozygous mutations in the ACAN gene, causes dominantly inherited short stature and, in many patients, early-onset osteoarthritis and degenerative disc disease. We aimed to further characterize this phenotypic spectrum with an emphasis on musculoskeletal health. Twenty-two individuals from nine families were enrolled. Histories and examinations focused on joint health, gait analysis, joint specific patient reported outcomes, and imaging studies were performed. All patients had dominantly inherited short stature, with the exception of a de novo mutation. Short stature was worse in adults versus children (median height −3.05 SD vs. −2.25 SD). ACAN mutations were not always associated with bone age advancement (median advancement +1.1 years, range 0 to +2 years). Children had subtle disproportionality and clinically silent joint disease—25% with osteochondritis dissecans (OD). Adults had a high prevalence of joint symptomatology–decline in knee function, disability from spinal complaints, and lower physical activity on outcome measures. Osteoarthritis (OA) and OD was detected in 90% of adults, and orthopedic surgeries were reported in 60%. Aggrecan deficiency leads to short stature with progressive decline in height SD, mild skeletal dysplasia, and increasing prevalence of joint pathology over time. Optimal musculoskeletal health and quality of life can be attained with timely identification of pathology and intervention.
1 INTRODUCTION
Aggrecan is a proteoglycan in the extracellular matrix of physeal and articular cartilage. Aggrecan plays a critical role in mediating chondrocyte-chondrocyte and chondrocyte-matrix interactions through its ability to bind hyaluronan, thereby providing cartilage resilience (Kiani et al., 2002). A study examining three families with dominantly inherited short stature, advanced bone age, and early growth cessation revealed a causative heterozygous mutation in the aggrecan gene (ACAN), leading to aggrecan deficiency (Nilsson et al., 2014). Not only did this report re-categorize patients previously classified with idiopathic short stature, but it also demonstrated the impact of aggrecan deficiency on musculoskeletal health—one family was noted to have early onset osteoarthritis (OA) and osteochondritis dissecans (OD). The clinical spectrum of aggrecan deficiency was expanded in a follow-up report of 103 individuals from 20 different families, whereby OA and intervertebral disc disease was noted in over 50% of the families (Gkourogianni et al., 2017). To date there has been no apparent correlation between the type or location of ACAN mutation and the presence of joint disease (Gkourogianni et al., 2017). Given the retrospective nature of these reports, we aimed to evaluate the musculoskeletal health of children and their relatives with aggrecan deficiency in greater detail.
2 METHODS
2.1 Subjects
This study was approved by the Institutional Review Board of Cincinnati Children's Hospital Medical Center (CCHMC) and conducted in accordance with the Declaration of Helsinki and the ICH Good Clinical Practice guidelines (Universal Trial Number (UTN): U1111-1192-2334; ACAN protocol with FDA-IND: 135870). All participants or their legal guardians provided written informed consent, and when applicable, assent. Prepubertal children with aggrecan deficiency and their affected relatives participated in this study. The inclusion criteria were: a documented heterozygous mutation in the ACAN gene (defined as either a deletion of the entire gene or >one exon, in-frame insertion/deletion of >one amino acids, any truncating mutation, or a missense mutation that was predicted to be damaging, segregated with affected individuals within the family or was de novo, and absent in the Exome Aggregation Consortium Database). The probands were also enrolled in a recombinant human growth hormone (rhGH) treatment protocol, which is reported elsewhere (Muthuvel et al., 2021). Probands were ≥2 years old, had a bone age ≥chronological age, and a normal IGF-I concentration.
2.2 Anthropometrics
Standing heights were obtained in triplicate with a Harpenden stadiometer and reported as the average of measurements. Weights were obtained with an electronic scale. Baseline seated height, arm span, and head circumference were obtained, as well as pubertal staging in accordance with the Marshall and Tanner method (Marshall & Tanner, 1969, 1970). Heights, weights, and body mass index (BMI) were plotted on the Centers for Disease Control (CDC) and Prevention growth charts with respective z-scores calculated based on National Health and Nutrition Examination Survey (NHANES) data (Fryar et al., 2012; McDowell et al., 2009). Z-scores for arm span (AS), arm span for height (AS for HT), sitting height (SH), sitting height/height ratio (SH/HT), and occipital frontal circumference (OCF) were based on data from the atlas of Pediatric Morphometrics by W. Gerver and de Bruin (2001) via the Growth Analyzer RCT- Data Analysis® tool.
2.3 Gene sequencing
Sequencing of DNA isolated from saliva samples was performed at both the Cincinnati Center for Growth Disorders and Children's National Laboratories. Coding exons 2–19 and exon-intron junctions of the ACAN gene were sequenced by Sanger. Primers were utilized as previously described by Stattin et al. (2010). Mutations were annotated using ACAN transcript NM_013227.3.
2.4 Musculoskeletal assessment
A sports medicine physician performed musculoskeletal assessments on each participant, including a history of joint complaints and prior surgeries as well as a comprehensive musculoskeletal exam. A physical therapist performed detailed three-dimensional (3D) walking gait analyses to evaluate the impact of ACAN mutation on gait mechanics. Three-dimensional gait analyses were performed on pediatric individuals with heterozygous mutations in ACAN who were ≥9 years of age and able to comply with a standardized treadmill walking and running routine, on affected adult relatives, and healthy adult controls who were recruited from a convenience sample of hospital employees. Three-dimensional stance phase walking kinematics were captured at 250 Hz with four, posteriorly mounted high-speed cameras (Bonita, Vicon Motion Systems, Ltd., Oxford, UK) using the 3D Gait system (Running Injury Clinic, Inc.; Calgary, Alberta, CA). Prior to walking, reflective anatomical markers (9.5 mm Pearl Markers, B & L Engineering, Santa Ana, CA) and tracking clusters (Running Injury Clinic, Inc.; Calgary, Alberta, CA) were affixed to each participant. Anatomical markers were placed on the bilateral medial malleoli, lateral malleoli, medial knee joint line, lateral knee joint line, and bilateral greater trochanters. Tracking shells with reflective markers were placed on the posterior pelvis, bilateral femurs, and bilateral shanks. Tracking markers were also affixed to the bilateral heel counters of the participants' preferred athletic footwear. The software system was calibrated according to manufacturer specifications, and each participant underwent a static calibration trial per the manufacturer guidelines (Running Injury Clinic, Inc., Calgary, Alberta Canada). After calibration, anatomical markers were removed and tracking markers and clusters remained. Participants then walked on a noninstrumented motorized treadmill (Bari-Mill, Woodway, Inc., Waukesha, WI) for approximately 5 min at their preferred speed in order to acclimate. Treadmill speed was then modified to match their typical community ambulation pace. An additional 2 min of acclimatization was provided. Afterward, 3D kinematics were collected for 30 s. Proprietary algorithms for automatic event detection and 3D kinematic calculation were utilized for analysis of data. These methods have been previously established as valid and reliable (Osis et al., 2016).
2.5 Patient reported outcome measures
Validated PRO measures were administered, including the Pediatric International Knee Documentation Committee (Pedi-IKDC) questionnaire, MARX Activity Rating Scale (MARX), Pediatric and Young Adult Quality of Life questionnaires (PedsQoL and Young Adult QoL), and Oswestry Disability Index (ODI). The Pedi-IKDC questionnaire is a knee-specific PRO to assess (1) symptoms (knee pain, stiffness, swelling, and weakness), (2) activity (climbing stairs, sitting up, squatting, and jumping), and (3) knee function. Scaled scores range from 0 to 100 with higher scores representing higher levels of function. The MARX aims to assess an individual's general activity level. The frequency of four functions (running, deceleration, cutting, and pivoting) common to most sports at one's healthiest, most active state within the past year are reviewed. A maximum scaled score is 16 points, with higher scores indicating more frequent participation in activity. The ODI is the gold standard assessment tool of lower back function, aimed to assess the impact of low back pain on activities of daily living. Scores range from 0 to 100 with a higher score indicating increased disability. The PedsQoL, patient and parent-proxy versions, assess patients' and parents' perceptions of health related QoL with a focus on physical, emotional, social, and academic functioning in pediatric patients with chronic health conditions. The Young Adult QoL is similar and was distributed to the affected adult cohort. Total scores range from 0 to 100 with higher scores indicating better QoL. Mean scores for each PRO were calculated and student T tests (unpaired, two-tailed heteroscedastic) were utilized to compare results of both the pediatric and adult cohorts. p-values were generated with ≤0.05 indicating a statistically significant difference.
2.6 Imaging
Radiographs of the left hand and wrist were obtained on probands and interpreted based on Greulich and Pyle standards (Greulich & Idell Pyle, 1959). Dual-energy x-ray absorptiometry (DXA) scans of the total body (TB) less head and lumbar spine (LS) were done on all probands with a Hologic QDR-4500A densitometer (Hologic Inc., Bedford, MA). Software (Apex) from Hologic Inc. was utilized for scan analysis and bone mineral density (BMD) z-scores were calculated using an FDA-approved pediatric reference database (Kalkwarf et al., 2013; Kelly et al., 2005; Zemel et al., 2011), and height-adjusted using an online BMD calculator (https://zscore.research.chop.edu/bmdcalculator). Bilateral four-view knee radiographs were done on all participants. Philips 3 Tesla Ingenia MRI of the right knee was done on participants aged 5–20 years to assess for early degenerative changes of the knee joint, including gross morphologic changes as well as microstructural cartilage changes. T2 relaxation time mapping was utilized, as this is highly sensitive to changes in collagen content and anisotropic orientation of collagen fibers within cartilage (Mosher & Dardzinski, 2004; Watrin et al., 2001). Additionally, T1ρ imaging, highly sensitive to the interaction of water molecules with glycosaminoglycan, provided further information about hyaline cartilage (Akella et al., 2001; Duvvuri et al., 1997; Regatte et al., 2006; Souza et al., 2013; Takayama et al., 2013). All imaging was interpreted by a centralized, board-certified radiologist.
3 RESULTS
3.1 Clinical features and anthropometrics
Baseline characteristics of 22 recruited study subjects (12 females), including 12 children (2–12 years) and 10 adults (25–62 years), are detailed in Tables 1 and 2. The median height standard deviation (SD) of the pediatric group was −2.25 SD (range −0.3 to −4.3) and median BMI 17.75 kg/m2, +1.2 SD (range 15.9–33.3 kg/m2, −0.4 to +2.8 SD). The median height SD for the adult group was −3.05 (range −2.2 to −4.4) and median BMI 25.8 kg/m2, +0.9 SD (range 21.7–39.9 kg/m2, 0 to +2.1 SD). There was subtle disproportionality with a median sitting height/height ratio of +2.2 SD (range −2.4 to +7.5) in the pediatric group, although with marked interindividual variability. However, upper extremity length was preserved: median AS for height (HT) of +1.3 SD (range +0.3 to +2.2) in the pediatric group and −0.3 SD (range −1.1 to +2.3) in adults (W. J. M. Gerver et al., 2020). Head circumference was normal in both the pediatric (median +0.3 SD, range −1.9 to +1.6) and adult (median −0.1 SD, range −1.3 to +1.6) cohorts.
| Family | Age y/m, sex | BA | WT, SD | BMI, SD | HT, SD | SH/HT, SD | AS for HT, SD |
|---|---|---|---|---|---|---|---|
| I | 7 y5 m, F | 9 y5 m | −0.7 | +1.2 | −3.1 | 0.5 (−2) |
N/A |
| II | 3 y5 m, M | 5 y0 m | +0.3 | +2.5 | −2.2 | 0.6 (+1.6) |
+1.5 |
| 5 y0 m, M | 6 y0 m | +1.5 | +2.8 | −1.1 | 0.6 (+3.3) |
+1.1 | |
| 12 y5 m, M | 13 y6 m | +2.3 | +2.4 | −0.3 | 0.6 (+7.5) |
+0.3 | |
| III | 6 y0 m, M | 7 y0 m | −3.1 | +0.8 | −4.3 | 0.6 (+3.9) |
+1.3 |
| IV | 4 y2 m, F | 5 y9 m | 0 | +1.2 | −1.2 | 0.5 (−2.4) |
+2.1 |
| 6 y11 m, F | 7 y10 m |
−0.6 | +0.9 | −2.3 | 0.6 (+4.3) | +1.8 | |
| V | 4 y1 m, M | 5 y6 m |
−0.2 | +1.2 | −1.5 | 0.6 (+2.8) | +1.2 |
| VI | 8 y1 m, F | 10 y0 m | −0.8 | +1 | −3 | 0.5 (−1.8) | +2.2 |
| VII | 11 y5 m, M | 12 y6 m |
−1.2 | +0.1 | −2 | 0.5 (−1.5) | +1.2 |
| VIII | 2 y5 m, F | 2 y6 m |
−1.1 | +1.2 | −2.6 | 0.6 (−0.1) | +2.1 |
| IX | 9 y8 m, F | 8 y10 m | −2.5 | −0.4 | −3.5 | 0.6 (+6.6) | +1.1 |
| Medians | 6.5 y | 7.4 y | −0.65 | +1.2 | −2.25 | 0.6 (+2.2) | +1.3 |
- Abbreviations: AS, arm span; BA, bone age; BMI, body mass index; HT, height; SD, standard deviation; SH, sitting height; WT, weight; y/m, years/months.
| Family | Age yrs, sex | WT, SD | BMI, SD | HT, SD | SH/HT | AS for HT, SD |
|---|---|---|---|---|---|---|
I |
25, F | +0.4 | +1.7 | −3.7 | 0.6 | N/A |
| 46, F | +0.9 | +2 | −4.4 | 0.5 | N/A | |
II |
35, F | +1.9 | +2.1 | −2.2 | 0.5 | −0.6 |
III |
35, M | −1.5 | +0.4 | −3.4 | 0.6 | −0.3 |
IV |
40, M | −0.1 | +1 | −2.2 | 0.5 | −1.1 |
VI |
42, F | −0.4 | +1.1 | −3.1 | 0.5 | +2.3 |
| 62, F | −0.7 | +0.8 | −2.8 | 0.5 | 0 | |
VII |
51, F | −1.4 | 0 | −2.3 | 0.5 | +1.1 |
VIII |
44, M | −1.8 | 0 | −3 | 0.5 | −0.3 |
IX |
46, M | −1.5 | +0.4 | −3.5 | 0.6 | −0.6 |
| Medians | −0.55 |
+0.9 | −3.05 | 0.5 | −0.3 |
- Abbreviations: AS, arm span; BMI, body mass index; HT, height; SD, standard deviation; SH, sitting height; WT, weight; yrs, years.
Three children (25%) had brachydactyly with webbing between their fingers, and one individual had midface hypoplasia (8%). The median bone age advancement in children was +1.1 years (range 0 to +2 years). A 12-year-old male from Family II had been on rhGH and anastrozole for 1 year prior to the study and a 4-year-old male from Family V had been previously treated with rhGH for 6 months.
3.2 ACAN genetic variants
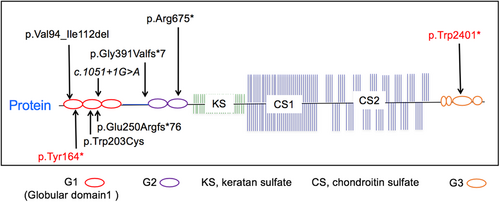
Mutations of this study's participants are listed in Table 3, two of which have been previously reported. Eight unique mutations were present within the nine families enrolled. Five of the mutations were located in the G1 region, one in the interglobular domain, two in the G2 region, and one in the G3 region (Figure 1). Of the mutations, four were nonsense, two were frameshift, one was missense, one was an in-frame deletion of exon 3, and one was a predicted in-frame excision of exon 6. All were predicted to be damaging to protein function. The proband of Family V, was noted to have a de novo mutation.
| Family | Exon | cDNA | Protein | Mutation type |
|---|---|---|---|---|
| I | 4 | c.492C>A | p.Tyr164* | Nonsense |
| II | 3 | c.280_336del | p.Val94_Ile112del | In-frame deletion |
| III | 4 | c.609G>T | p.Trp203Cys | Missense |
| IV | 16 | c.7202G>A | p.Trp2401* | Nonsense |
| V | 10 | c.2023C>T | p.Arg675* | Nonsense; de novo |
| VI | Intron 6 | c.1051+1G>A | p.Val255_Glu352del | Predicted in-frame excision of exon 6 |
| VII | 5 | c.748del | p.Glu250Argfs*76 | Frameshift |
| VIII | 7 | c.1172delG | p.Gly391Valfs*7 | Frameshift |
| IX | 10 | c.2023C>T | p.Arg675* | Nonsense |
- Note: Mutations of families I and IV have been previously reported (Gkourogianni et al., 2017).
3.3 Musculoskeletal assessment and imaging
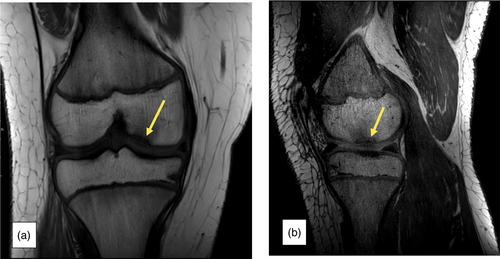
In the pediatric group, no joint complaints were reported. On exam, five (42%) children had pes planus, two (17%) had a positive patellar grind test suggestive of patellofemoral (PF) syndrome, and one (8%) had genu recurvatum. Knee radiographs were normal in nine of the children (75%), one child (8%) had OD, and two (17%) had osteochondral lesions (OLs). One of the OL lesions was then qualified as “unstable OD” on MRI (Figure 2a,b). Knee MRIs revealed a total of three children with OD (25%). DXA scans of TB and LS demonstrated normal BMD in all pediatric individuals (Table 4).
| Family | Age yrs, sex | Musculoskeletal exam | Knee X-ray | Knee MRI | TBLH BMD, HAZ | LS BMD, HAZ |
|---|---|---|---|---|---|---|
| I | 7, F | Pes planus, genu recurvatum, pronation of feet | Normal | Normal | +0.1 | 0 |
| II | 3, M | N/A | Normal | N/A | −0.4 | +1 |
| 5, M | N/A | Normal | N/A | +0.5 | +2.2 | |
| 12, M | Pes planus | OL of the patella versus dorsal defect, OSD of the knee | Unstable OD of medial femoral condyle | Not done | Not done | |
| III | 6, M | Normal | OL of lateral femoral condyle | Partial discoid lateral meniscus, femoral condyle subchondral irregularities (NCS) | 0 | −0.5 |
| IV | 4, F | Pes planus | Normal | N/A | −0.8 | −0.4 |
| 6, F | Pes planus | Benign fibrous cortical defect of distal femur diaphysis (NV) | Distal femur fibrous cortical defect (NV) | +0.6 | +1.4 | |
| V | 4, M | N/A | Normal | N/A | +0.1 | +0.1 |
| VI | 8, F | Normal | Normal | OD of patellar apex and femoral sulcus | −0.7 | −0.2 |
| VII | 11, M | Pes planus, positive patellar grind | Large OD of patellae with near complete fragmentation | OD of patella | −0.3 | −0.8 |
| VIII | 2, F | N/A | Normal | N/A | 0.378a | 0.454a |
| IX | 9, F | Positive patellar grind | Normal | Horizontal tear of lateral meniscus, posterior horn | +0.2 | +0.3 |
- Abbreviations: BMD, bone mineral density; HAZ, height adjusted z-score; LS, lumbar spine; NCS, not clinically significant; NV, normal variant; OD, osteochondritis dissecans; OL: osteochondral lesion; OSD, Osgood–Schlatter disease; TBLH, total body less head; yrs, years.
- a Patient VIII had a chronologic and bone age of 2 years old, so no reference data was available to calculate a HAZ score. Thus, BMD reported in terms of g/cm2.
In the adult group, knee pain was reported in eight individuals (80%) with onset in late adolescence to early twenties. Five (50%) had recurrent patellar dislocations. Six adults (60%) had back and hip pain, with onset between the second and sixth decade of life. Two adult males with back pain reported a diagnosis of ankylosing spondylitis. Six adults (60%) had prior orthopedic surgeries: four were knee surgeries, two were hip replacements, and two were back surgeries. On exam, 60% had a positive patellar grind test, 50% had pes planus, and 20% had valgus alignment. Eighty percent had OA on knee radiographs and one individual (10%) was noted to have OD of the right medial femoral condyle. Radiograph findings correlated well with reported symptomatology (Table 5).
| Family | Age yrs, sex | Joint history | Surgeries | Musculoskeletal exam | Knee X-ray |
|---|---|---|---|---|---|
| I | 25, F | Jaw clicking and back, hip, ankle, and hand pain in adolescence/early 20 s | None | Pes planus; knee pain/weakness; patellar and greater trochanteric tenderness; clicking of IT band | Normal |
| 46, F | Patellar dislocation and knee, back, and hip pain in 30 s | None | Pes planus; limping gait; greater trochanteric and patellar tenderness; patellar effusion; impaired external rotation of hip; hip weakness | Moderate unilateral knee OA | |
| II | 35, F | Unremarkable | None | Pes planus; valgus alignment; patellar grind | Valgus alignment, moderate BL OA, lateral patellar subluxation and tilting suggesting PF instability |
| III | 35, M | Ankylosing spondylitis and back, shoulder, neck, knee, and foot pain in 20 s; patellar dislocation since adolescence | Lateral release and meniscal repair in 20 s; back surgery in 30 s | Patellar grind; anterior knee pain with squat/compression | Mild PF OA |
| IV | 40, M | Ankylosing spondylitis and shoulder, back, knee, and foot pain in 20 s | Back surgery in 30 s; knee surgery in 20s | Patellar grind; anterior knee pain with squat/compression | Prior avulsion injury of the fibular head, early R PF OA |
| VI | 42, F | Patellar dislocation, OD, and knee pain in adolescence; hip pain in 30 s | Hip replacement at 35 yrs | Unremarkable | Unilateral OD of medial femoral condyle, mild PF OA |
| 62, F | Knee, neck, shoulder, and hip pain in 50/60 s | Hip replacement at 53 yrs | Valgus alignment; pes bursa tenderness; patellar grind | PF OA (R > L) | |
| VII | 51, F | Hip, ankle, knee, and back pain in 30 s | None | Patellar tenderness; patellar grind; foot pain/weakness | Mild PF OA |
| VIII | 44, M | Trochlear dysplasia, lateral displacement of patellae with dislocation, and knee pain in teens/20 s | BL trochlear osteotomies and lateral release in 20 s | Pes planus; hip weakness; knee swelling; −3° knee flexion; patellar grind | Severe PF OA, irregular shaped sclerotic margin of the tibial tuberosity |
| IX | 46, M | BL meniscal tears and patellar dislocation in teens; knee pain since 20 s; hand arthritis and back and hip pain in 40 s | BL meniscal repair in teens; knee arthroscopies for loose bodies | Pes planus; hip weakness; patellar tenderness | BL mild to moderate OA, lateral patellar subluxation and tilting suggesting PF instability |
- Abbreviations: BL, bilateral; IT, iliotibial; L, left; OA, osteoarthritis; OD, osteochondritis dissecans; PF, patellofemoral; R, right; yrs, years.
3.4 Gait analysis
It was decided a priori to only analyze the walking kinematics of participants and healthy controls 9 years of age and older, as the reliability of this test in the younger population was uncertain given their lack of previous exposure to treadmill use. Three pediatric participants (Table 6), all 10 adult family members, and 10 adult controls were studied.
| Family VII | Family II | Family IX | ||||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | p-value | ||
| Knee | ||||||||
| SAG | IC | 0.0 | −3.9 (E) | 2.4 | −0.2 | 7.4 | 5.3 | |
| MST | 46.6 | 48.6 | 44.0 | 45.0 | 66.5 | 61.4 | ||
| TO | 46.6 | 48.6 | 44.0 | 45.0 | 66.5 | 61.4 | ||
| COR | IC | −3.3 (ABD) | −3.3 | 3.1 | −4.2 | −3.8 | −2.4 | |
| MST | −1.7 | −2.8 | −0.3 | −0.3 | −9.4 | −2.6 | ||
| TO | −5.6 | −6.7 | −3.7 | 0.9 | −9.4 | 1.1 | ||
| TR | IC | −11.9 (ER) | −26.1 | −10.4 | 0.7 | −11.9 | −8.3 | |
| MST | 4.4 | −9.7 | 11.4 | 11.5 | −9.4 | −2.6 | ||
| TO | 3.8 | −9.7 | 11.4 | 11.5 | 1.5 | −1.9 | ||
| Hip | ||||||||
| SAG | IC | 22.8 | 21.1 | 27.8 | 25.6 | 31.0 | 23.4 | |
| MST | 23.0 | 21.8 | 27.8 | 25.8 | 31.1 | 23.4 | ||
| TO | −2.1 (E) | −1.8 | −7.4 | −9.3 | 20.3 | 14.3 | ||
| COR | IC | −5.0 (ABD) | −3.5 | 0.9 | 1.6 | 4.6 | −1.6 | |
| MST | 1.7 | 11.4 | 6.0 | 2.2 | 6.8 | 4.3 | ||
| TO | −8.6 | 1.6 | −1.6 | −4.0 | −0.4 | −5.4 | ||
| TR | IC | 4.8 | 13.9 | 5.4 | −4.0 | 6.4 | 2.1 | |
| MST | 7.1 | 16.0 | 10.7 | −1.0 | 9.4 | 4.5 | ||
| TO | −12.2 (ER) | −1.4 (ER) | −11.6 | −16.6 | −6.6 | 7.5 | ||
- Note: All values are in degrees.
- Abbreviations: COR, coronal plane (negative values = joint abduction; positive values = joint adduction); IC, initial contact (point of time when foot contacts ground); MST, midstance (point of time when center of mass is at its highest point during stance phase); SAG, sagittal plane (negative values = joint hyperextension; positive values = joint flexion); TO, toe-off (point of time when foot leaves the ground); TR, transverse plane (negative values = joint external rotation; positive values = joint internal rotation).
An 11-year-old male from Family VII had PROs consistent with normal musculoskeletal health, function, quality of life, and activity level (Pedi-IKDC score of 96.74, PedsQoL score of 100, and MARX score of 14). His knee MRI and radiographs demonstrated OD of patellae with near complete fragmentation. He walked at 1.05 m/s and demonstrated symmetry of bilateral spatiotemporal features (stride length, stance time, and swing time). He had no obvious lower extremity stance phase asymmetries in the sagittal plane. In the coronal plane he ambulated with increased right versus left hip adduction (11.4° vs. 1.7°). In the transverse plane, he ambulated with increased right versus left knee external rotation (9.7° vs. 4.4°) and increased right versus left hip internal rotation (16.0° vs. 7.1°). These mechanics resulted in an increased quadriceps angle, placing additional stress on the patellofemoral joint of the right limb compared to the left. This increases the theoretical likelihood of anterior knee pain in the future (Powers et al., 2003).
A 12-year-old male from Family II had PROs consistent with normal musculoskeletal health, function, and quality of life, but notable for limited activity (Pedi-IKDC score of 100, PedsQoL score of 100, and MARX score of 9). His knee MRI demonstrated unstable OD of the medial femoral condyle. He walked at 1.22 m/s and demonstrated symmetry in bilateral spatiotemporal features. He demonstrated no sagittal plane asymmetries. As with the patient from Family VII, he demonstrated significant coronal and transverse plane asymmetries, particularly at the hip. During stance, the left hip was significantly more adducted (6.0° vs. 2.2°) and internally rotated (10.7° vs. 1.0°) than the right. His left knee was more externally rotated than his right (−10.4° vs. 0.7°). The net effect is an increase in the quadriceps angle, predisposing to anterior knee pain.
A 9-year-old girl from Family IX had PROs consistent with normal musculoskeletal health, function, and quality of life, as well as the highest activity score for the pediatric cohort (Pedi-IKDC score of 100, PedsQoL score of 92, and MARX score of 16). Knee MRI and radiographs were normal. Self-selected walking speed at 0.7 m/s was significantly slower than the other pediatric participants. She had no spatiotemporal asymmetries. She showed increased amounts of bilateral stance phase knee and hip flexion. This may have been a result of her significantly shorter stride lengths (0.7 m vs. 1.05 m and 1.2 m, respectively). She did not demonstrate significant side-to-side sagittal plane asymmetries apart from her stance phase knee flexion and hip flexion, which were respectively 5° and 8° more on the left. At the knee level, she demonstrated increased left knee abduction and external rotation. At the hip level she demonstrated increased left hip adduction and internal rotation. Cumulatively, these findings may also increase left PF stress.
Affected adult relatives were significantly shorter (1.48 m ± 0.09 m vs. 1.72 m ± 0.09 m; p <0.01), lighter (57.6 kg ± 7.1 kg vs. 73.8 kg ± 10.4 kg), and ambulated with a shorter stride length (1.1 m ± 0.1 m vs. 1.2 m ± 0.07 m) than adult controls. There were no other significant stance phase kinematic differences between affected adult relatives and controls with the exception of stance-related coronal plane (left) knee abduction, which was significantly more abducted (p = 0.03) for controls compared to affected adult relatives (14.7° ± 3.2° vs. 10.2 ± 5.0°).
3.5 Musculoskeletal PROS
Pedi-IKDC scores in our pediatric study population (mean 96 ± 8.6) were like those reported for healthy pediatric norms (mean 86.7 ± 16.8; median, 94.6; 34% of scores reached the ceiling value of 100). However, scores for the 10 adult participants (mean 75 ± 19) were significantly lower than those from nonsurgical, age, and sex matched unaffected adults (mean 84.7–95.5 ± 8.2–16.2), indicating more symptoms and poor overall function in our adults with aggrecan deficiency (Anderson et al., 2006; Nasreddine et al., 2017). The minimal detectable change has been reported to be in the 9–16 range for adults and 12 for pediatric IKDC (McHugh et al., 2020). Similarly, the ODI scores for low back pain and function were 0.22 ± 0.67 in pediatric subjects, indicating no disability. Adult subjects reported a mean of 19.4 ± 17.5, consistent with moderate disability. As predicted, MARX activity scores were significantly higher in pediatric subjects, demonstrating increased physical activity levels compared to adult study subjects (pediatric mean 12.5 ± 2.4; adult mean 3.2 ± 4.6; p <0.001). Additionally, the adult scores were similar to patients that have sustained serious knee injury (Letchford et al., 2012). This inverse correlation with age has been described and predicted by both lifestyle alterations as well as possible joint dysfunction and other factors. Pediatric QoL inventory surveys were completed by both the pediatric participant and their parent-proxy. Pediatric scores demonstrated a favorable QoL: patient reported mean was 88.1 ± 12.6 compared to historical norms of children from California (83.3 ± 13.5), and parent-proxy reported mean was 85.5 ± 16.7 compared to a mean of 78.9 ± 16.6 seen among general population norms (Varni et al., 2003). Affected adults personally completed the young adult QoL survey with mean scores of 76.7 ± 15.7, which is lower than that reported in healthy young adult populations (85.9 ± 11.2), but similar to young adult populations with chronic disease (75.23 ± 15.1; Limperg et al., 2014).
4 DISCUSSION
Our study provides the first detailed characterization of the musculoskeletal phenotype in individuals affected by heterozygous ACAN mutations. Previous reports mention early onset OA, OD, and intervertebral disc disease in these patients, but were limited by their retrospective nature (Gkourogianni et al., 2017; Nilsson et al., 2014). We recruited 22 individuals from nine families with eight unique ACAN mutations to undergo detailed histories, comprehensive musculoskeletal examinations, patient reported outcome measures, 3D gait analyses, and targeted imaging to better elucidate the prevalence, nature, and degree of joint pathology.
A minority of our patients presented with mild dysmorphic features: brachydactyly, increased webbing between fingers, and midface hypoplasia. The observation in the children with mild skeletal disproportionality corresponds to prior reports, demonstrating an increased SH/HT ratio, but an AS similar to HT (W. J. M. Gerver et al., 2020; Gkourogianni et al., 2017). All patients had dominantly inherited short stature, except for the one with a de novo mutation. The short stature phenotype was worse in the adults due to the premature growth cessation associated with rapid bone age advancement. The effect on adult height achievement appears unrelated to the underlying mutation, suggesting a common mechanism impairing growth plate chondrogenesis.
In contrast to initial reports, but in alignment with more recent observations (Hu et al., 2017; Xu et al., 2018), our study also demonstrated that ACAN mutations are not always associated with bone age advancement. The rate of bone age maturation will be of particular importance in patients undergoing growth promoting treatment to better understand its impact on adult height outcome. Our group is currently conducting a prospective trial utilizing rhGH in prepubertal patients with aggrecan deficiency. Further studies are also needed to identify underlying factors, including genetic variants, that may drive bone age advancement in select individuals.
Musculoskeletal assessment yielded several anomalies: pes planus, patellar grind suggestive of PF syndrome, as well as valgus alignment with recurrent patellar dislocations in adults, none of which have been previously described. Gait assessment within our pediatric cohort demonstrated variations in hip and knee alignment that predispose to PF stress. In children, we postulate that the asymmetric gait mechanics may be due to OD noted in the first two males, as a protective mechanism to off-load weight from the more affected joint; however, this cannot be definitively concluded as MRIs were taken of the right knee only. Additionally, it remains unclear if these gait asymmetries are a direct result of their underlying aggrecan defect and its impact on articular cartilage, as even the patient without findings of OD and completely normal knee radiographs demonstrated it. Ultimately, these mechanics are clinically significant as it creates abnormal joint loading and PF stress, predisposing them to future PF pain syndrome (Farrokhi et al., 2011). For the affected adults, their decreased abduction at the knee may be a compensatory mechanism to reduce stress on the patellofemoral (PF) joint, as 90% of our population had patellofemoral OA or OD.
Pediatric participants remained essentially asymptomatic and had favorable joint-related patient reported outcomes. In contrast, joint pathology was common among adults (90% with at least one joint complaint and 60% requiring a major orthopedic surgery as early as their adolescent years). The most common site for pain was the knee (80%), followed by the back (60%) and hip joints (60%). In line with prior reports, OA of the knees presented as early as in (late) adolescence, whereas hip and back pain developed after the second decade of life (Gkourogianni et al., 2017). Comparatively, the lifetime risk of symptomatic knee OA in the general population is approximately 40-50% with incidence rates sharply rising after age 50 (Neogi & Zhang, 2013). ODI scores indicated no disability in pediatric subjects versus moderate disability in adults. At this time, we are unable to correlate these findings to the degree of vertebral disc disease seen on imaging. Given that intervertebral joints contain many normal variants, we felt that discerning between true pathology versus normal variation would be difficult on MRI within our limited cohort; therefore, future studies can consider additional imaging modalities to further evaluate this.
Two adult males in our study reported a diagnosis of ankylosing spondylitis. Although the exact cause of ankylosing spondylitis is unknown, extensive progress has been made in identifying susceptibility alleles for the disease, of which human leukocyte antigen B27 (HLA-B27), located on chromosome 6, has been shown to confer the greatest genetic risk. More recently, variants of the M1-aminopeptidase gene, ERAP1 and ERAP2, located on chromosome 5, and killer immunoglobulin like receptors (KIRs) encoded within the lymphocyte receptor complex on chromosome 19 have also been associated with ankylosing spondylitis (Hanson & Brown, 2017). None of these loci are in proximity to the ACAN gene on chromosome 15q26; therefore, no obvious genotype–phenotype correlation can be made at this time.
Imaging studies revealed normal bone mineral density among our pediatric participants. Three children (25%) had OD of the knee despite being asymptomatic. 80% of adults had OA on knee radiographs and one individual had OD. Adults with joint pathology on imaging had corresponding joint pain and PROs demonstrating significant decline in knee function. Additionally, we report that joint pathology including OD can be present with mutations throughout the gene. Prior reports only note mutations in the C-type lectin domain among individuals with OD (Gkourogianni et al., 2017). However, in our study, all three children and one adult with OD had early truncating mutations that should result in haploinsufficiency of the ACAN gene. Further in vivo studies will have to be done to demonstrate how haploinsufficiency of ACAN leads to OD.
In summary, our study provides a detailed description of the musculoskeletal phenotype of individuals with heterozygous ACAN mutations. This phenotype includes short stature with progressive decline in height SD over time, mild skeletal dysplasia, clinically silent OD in youth, and an increasing prevalence of joint pathology in early adulthood often requiring corrective surgery. The high prevalence of joint disease with worsening function over time underscores the importance of a timely diagnosis. These individuals will likely present to their primary care provider for either joint complaints or short stature. They are often referred to rheumatology, sports medicine, orthopedics, physical therapy, or endocrinology for additional evaluation. Clinicians should be suspicious for the presence of aggrecan deficiency when evaluating patients with joint complaints who are also short. Alternatively, ACAN evaluation should be considered for short stature in the setting of a strong personal or family history of joint pathology. If an ACAN mutation is identified, then detailed musculoskeletal assessments, close monitoring of joint-related symptoms, and imaging of knee joints should be performed. As evidenced by our study, only one of the three pediatric cases of OD was apparent on four-view knee radiograph. Therefore, it may be prudent to perform baseline MRIs in children that can cooperate for the examination. Last, these patients require counseling on conservative measures, including joint strengthening and low impact exercises, to preserve joint function over time, diminish the need for orthopedic treatments, and preserve quality of life.
ACKNOWLEDGMENT
This study was supported by an investigator-initiated grant from Novo Nordisk, Inc. Novo Nordisk played no role in the design, conduct, data analysis, or manuscript preparation.
CONFLICT OF INTEREST
There are no prior publications or submissions with any overlapping information. Philippe Backeljauw has received grant/research support from Novo Nordisk, Ipsen, and Opko, as well as consulting fees from Novo Nordisk, Ipsen, Endo Pharmaceuticals, Ascendis, BioMarin, and Sandoz. Andrew Dauber has received consulting fees from Biomarin, Novo Nordisk, Sandoz, Pfizer, Ipsen, Ascendis, and OPKO Biologics. He has received research support from Biomarin, Novo Nordisk, Ipsen, and Pfizer.
AUTHOR CONTRIBUTIONS
Andrew Dauber and Philippe Backeljauw conceptualized and designed the study. All authors participated in acquisition of the study data. Eirene Alexandrou facilitated with execution of the study, and data collection and interpretation, as well as drafted the manuscript. All authors critically reviewed the manuscript and approved the final manuscript as submitted.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.