Extending the phenotype of posterior column ataxia with retinitis pigmentosa caused by variants in FLVCR1
Abstract
Posterior column ataxia with retinitis pigmentosa (PCARP) is a rare autosomal recessive condition due to variants in the Feline Leukemia Virus Subgroup C Cellular Receptor 1 (FLVCR1) gene which was first described in 1997. In this article, we describe a young female patient with a childhood diagnosis of retinitis pigmentosa and learning disability, presenting with progressive ataxia from her late teens. Examination revealed spastic lower limbs with absent reflexes, and reduced vibration and joint position sensation. Magnetic resonance imaging showed normal cerebellar volume and linear signal abnormality within the posterior columns of her spinal cord. Trio exome analysis confirmed two variants in FLVCR1. Our case extends the phenotype of PCARP to include learning disability and developmental delay, and highlights the importance of considering this rare condition in young adults or children with visual impairment and ataxia.
1 BACKGROUND
The autosomal recessive syndrome of posterior column (sensory) ataxia with retinitis pigmentosa (PCARP) was first described by Higgins et al in 1997 (Higgins et al., 1997). In 2010, genetic analysis identified variants in the Feline Leukemia Virus Subgroup C Cellular Receptor 1 (FLVCR1) gene in 14 patients from three families with PCARP. The syndrome consists of early visual loss or night blindness from retinitis pigmentosa (RP) associated with progressive ataxia. To date, only 42 patients and 19 different variants have been described (Beigi et al., 2020; Castori et al., 2017; Chiabrando et al., 2016; Glöckle et al., 2014; Ishiura et al., 2011; Kuehlewein et al., 2019; Lee et al., 2018; Liu et al., 2015; Puffenberger et al., 2012; Rajadhyaksha et al., 2010; Shaibani et al., 2015; Tiwari et al., 2016; Yusuf et al., 2018).
FLVCR1 gene codes for a mammalian cell heme exporter. It is hypothesized that it protects erythroid progenitors from potential heme toxicity during the heme synthesis phase of erythropoiesis. It is ubiquitously expressed at potential sites of heme trafficking, for example, the liver and small intestine but expression has also been seen in the brain, kidney, lung, spleen, uterus, and placenta (Khan & Quigley, 2013; Quigley et al., 2004). Expression is reported to be particularly high in the retina, followed closely by the posterior columns of the spinal cord (Rajadhyaksha et al., 2010). Murine embryos with knockout of FLVCR1 display a phenotype similar to patients with Diamond–Blackfan anemia, a congenital syndrome that includes red cell aplasia and elevated red cell adenosine deaminase levels (Lipton & Ellis, 2009).
2 CASE REPORT
A 23-year-old right-handed woman transitioned from pediatric to adult neurology services. She was born at full term after a normal pregnancy. She exhibited speech delay and motor delay, walking at 2½ years. Her parents reported that she was always clumsy growing up and had early night blindness. She was diagnosed with RP at 6 years old. She attended mainstream school but was diagnosed with a mild learning disability at the age of 12. She was diagnosed with scoliosis in her late teens. She was first seen by neurology at 20 years old due to slowly progressive balance difficulties causing falls on uneven ground and difficulty standing up from sitting. She is the youngest of three children of non-consanguineous parents. Both parents and siblings are unaffected and there is no history of neurological problems in the family.
On examination, the patient had a narrow facial appearance. Her cranial nerve examination was unremarkable. She had a bilateral upper limb drift. She had moderate spasticity in her lower limbs but there was no limb weakness. Her deep tendon reflexes were absent. She walked with a crouched, narrow-based, unsteady gait. She had difficulty walking in tandem. Romberg's test was positive. She was dysarthric and had an intention tremor but no past pointing. Sensory examination revealed markedly reduced vibration and joint position sense. She had mild scoliosis but systemic examination was otherwise normal.
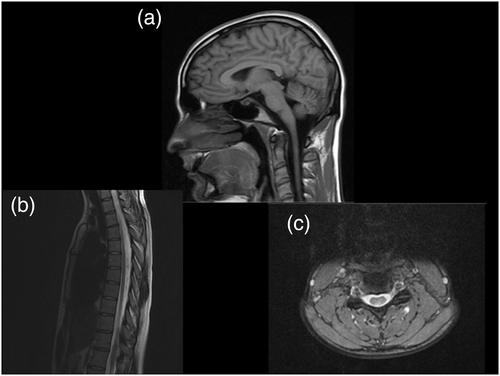
Routine laboratory tests (full-blood count, renal, and liver function), creatinine kinase, lactate, amino acids, very long-chain fatty acids, and microarray were all normal. MRI brain was normal, including normal cerebellar architecture. Her MRI spine revealed signal abnormality within her posterior columns (Figure 1). Neurophysiology testing showed absent sensory responses, consistent with a severe sensory axonal polyneuropathy.
Given the slowly progressive syndrome starting in childhood, genetic causes were considered. The clinical phenotype of retinitis pigmentosa in combination with a sensory ataxia was recognized to be most in keeping with PCARP. Genetic sequencing of the FLVCR1 gene performed by Centogene identified two variants; a c.1022A>G (p.Tyr341Cys) missense variant in exon 3 and a c.1307+5G>T splice site variant in intron 6. The missense variant is located in a moderately conserved nucleotide and highly conserved amino acid position. It is reported in the Exome Aggregation Consortium with a frequency of 0.00003%. The exome sequencing project describes it with a frequency of 0.0001% in the European American population and 0.0002% in the African American population. This variant is classified as likely pathogenic according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology Consensus Guidelines (Richards et al., 2015). The splice site variant is located in a highly conserved nucleotide and is reported in the Exome Aggregation Consortium with a frequency of 0.000008%. To date, this variant has not been described in the Exome Sequencing Project or the 1000 Genome Browser. This variant is of uncertain significance. Segregation analysis revealed that the patient's father was a heterozygous carrier of the c.1022A>G (p.Tyr341Cys) variant and her mother was a heterozygous carrier of the c.1307+5G>T variant. Focused whole-exome sequencing, performed by an accredited commercial genetics provider, identified the same heterozygous variants in FLVCR1 but no other genetic abnormalities were identified to explain the ataxia, sensory neuropathy or developmental delay, suggesting that these variants are causative. In specific, mtDNA analysis for NARP (Neuropathy, Ataxia, and Retinitis Pigmentosa) was normal.
3 DISCUSSION
Here, we describe a patient with the cardinal features of PCARP syndrome (sensory ataxia and retinitis pigmentosa) who is compound heterozygous for two variants in the FLVCR1 gene. We support the extension of the clinical phenotype to include learning disability and developmental delay.
The patients from the original three families described in the literature all had retinitis pigmentosa with progressive balance impairment. Cerebellar features were absent or minimal. Deep tendon reflexes were absent and proprioception was impaired. Other features included scoliosis, gastrointestinal motility issues, and cataracts (Rajadhyaksha et al., 2010).
Since the original three families were described, more variants have been identified in the FLVCR1 gene and a spectrum of diseases has emerged. Reported variants in FLVCR1 can cause RP in isolation or in combination with other symptoms ranging from mild neurological deficit to a full PCARP syndrome or even a phenotype of hereditary sensory and autonomic neuropathy (HSAN). Of note, nine reported cases with isolated RP carry the c.1092+5G>A splice site variant on at least one allele (Glöckle et al., 2014; Tiwari et al., 2016; Yusuf et al., 2018). The c.1022A>G variant was reported in a homozygous state in seven patients with isolated RP (Dockery et al., 2019). It has also been noted by Kuehlewein et al. that all patients with PCARP are either homozygous or compound heterozygous for two missense variants or one missense and one frame-shifting variant (Kuehlewein et al., 2019).
Notably, anemia has not been described in human patients with PCARP. It has been proposed that this is due to erythroid progenitor heme export being reduced, but not absent (Khan & Quigley., 2013).
MRI features in PCARP are variable but usually include normal cerebellar structure and T2 hyperintensity in the posterior half of the spinal cord (Castori et al., 2017; Higgins et al., 1997; Ishiura et al., 2011). However, normal spinal imaging has also been reported (Beigi et al., 2020). Nerve conduction studies reported in five patients with PCARP, HSAN, and a phenotype overlapping between the two all showed a sensory axonal neuropathy (Castori et al., 2017; Chiabrando et al., 2016; Higgins et al., 1997). One patient was also found to have absent sympathetic skin responses, absent somatosensory evoked potentials, and a reduced blink reflex (Castori et al., 2017). Sural nerve biopsy in three patients with PCARP showed axonal degeneration with loss of large myelinated fibers (Rajadhyaksha et al., 2010), but in one patient with a PCARP/HSAN overlap syndrome there was loss of myelinated and unmyelinated fibers (Castori et al., 2017).
The proposed mechanism of the disease relates to dysfunction of the FLVCR1 heme exporter leading to increased reactive oxygen species and intracellular heme accumulation, which has been shown in in vitro studies (Castori et al., 2017). This results in an apoptotic cascade that causes the selective degeneration of cells with high FLVCR1 expression (Rajadhyaksha et al., 2010). Yusuf et al. suggested that splice site variants in FLVCR1 allow production of the protein at a level which is sufficient for tissues with lower expression (Yusuf et al., 2018). Given that FLVCR1 expression is very high in the retina and slightly lower in the posterior columns, this may explain why patients with these variants have RP without posterior column involvement. Missense variants, however, appear to disrupt the protein function more severely.
Our patient had an early diagnosis of RP with subsequent slowly progressive ataxia and posterior column dysfunction, in keeping with the previously reported phenotype of PCARP. Neuroimaging identified the previously reported T2 hyperintensity in the dorsal cord. Our case also supports the extension of the phenotype to include learning disability and developmental delay, as described in more recent reports (Beigi et al., 2020; Chiabrando et al., 2016). The two variants found in our patient have not previously been reported in any patient with the full PCARP syndrome. Segregation analysis confirmed that her unaffected parents are each heterozygous for one of these variants, suggesting that they are in fact pathogenic.
While PCARP remains a very rare entity, this case highlights the importance of including it in the differential diagnosis of children or young adults with visual impairment and ataxia.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.