Rare presentation of FDX2-related disorder and untargeted global metabolomics findings
Abstract
We present the case of a 20-year-old male with a history of myopathy and multiple episodes of rhabdomyolysis, and lactic acidosis. He needed hemodialysis for severe rhabdomyolysis-related acute renal failure at the time of initial presentation (age 10 years). Exome sequencing detected a homozygous likely pathogenic variant in FDX2 (c.12G>T, p.M4I). The FDX2 gene encodes a mitochondrial protein, ferredoxin 2, that is involved in the biogenesis of Fe–S clusters. Biallelic pathogenic variants in FDX2 have previously been associated with episodic mitochondrial myopathy with or without optic atrophy and reversible leukoencephalopathy. Only two cases with FDX2-related rhabdomyolysis as a predominant feature have been reported in medical literature. Here, we report a third patient with FDX2-related recurrent, severe episodes of rhabdomyolysis and lactic acidosis. He does not have optic atrophy or leukoencephalopathy. This is the oldest patient reported with FDX2-related disorder and he has significantly elevated CK during episodes of rhabdomyolysis. In addition, we describe untargeted global metabolomic findings during an episode of metabolic decompensation, shedding light on the biochemical pathway perturbation associated with this ultra-rare genetic disorder.
1 INTRODUCTION
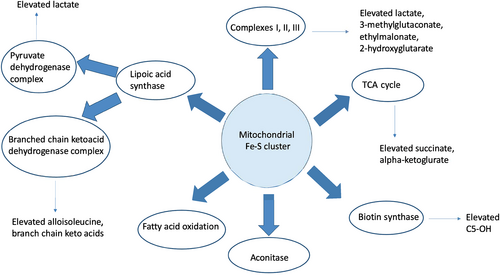
Ferredoxins are iron–sulfur proteins that act as electron donors and play an important role in many biochemical reactions (Shi et al., 2012). Humans have two ferredoxins; ferredoxin-1 encoded by FDX1 on chromosome 11q22.3 and ferredoxin-2 encoded by FDX2 on chromosome 19p13.2. Human mitochondrial ferredoxin-2 (FDX2) encoded by FDX2 (MIM*614585), previously known as FDX1L (ferredoxin-1-like protein), plays an important role as an electron donor and in iron–sulfur (Fe–S) cluster biosynthesis (Sheftel et al., 2010). Iron–sulfur (Fe–S) clusters are critical for multiple biological processes including mitochondrial respiration, electron transfer, synthesis of cofactors, DNA replication and repair as well as gene regulation (Cai et al., 2017; Stehling et al., 2014). Fe–S proteins also play an important role in biosynthesis of lipoic acid, which is a cofactor for several enzymes including the four alpha-ketoacid dehydrogenases (pyruvate dehydrogenase [PDH], α-ketoglutarate dehydrogenase [α-KGDH], 2-oxoadipate dehydrogenase [2-OADH], branched chain ketoacid dehydrogenase [BCKDH]), and the mechanistically similar glycine cleavage system (GCS) (Pain & Dancis, 2016). FDX2 deficiency belongs to the group of mitochondrial iron–sulfur cluster biogenesis disorders.
Biallelic pathogenic variants in FDX2 have been associated with mitochondrial myopathy with or without optic atrophy optic atrophy and reversible leukoencephalopathy (MEOAL) (MIM#251900). The characteristic clinical phenotype includes recurrent episodes of myalgia with progressive myopathy, early-onset optic atrophy, reversible leukoencephalopathy, axonal polyneuropathy and a variety of hematological and endocrine abnormalities including microcytic anemia, subclinical hypothyroidism, and diabetes mellitus (Gurgel-Giannetti et al., 2018). However, only two cases of isolated rhabdomyolysis associated with autosomal recessive FDX2-related disorder in a 12-year-old and 5-month-old individual have been previously reported (Lebigot et al., 2017; Spiegel et al., 2014).
Here, we present a third patient with FDX2-related disorder who presented with recurrent episodes of rhabdomyolysis and lactic acidosis. The degree of metabolic abnormality evident in this currently reported patient is much more severe than in previously reported cases. We describe this patient's clinical course and management over the past 10 years. In addition, untargeted metabolomic profiling results during an acute episode provide an improved understanding of the pathophysiology of this rare genetic disorder.
2 CASE REPORT
We describe a 20-year-old male who initially presented with severe rhabdomyolysis following an episode of streptococcal pharyngitis at the age of 10 years. Rhabdomyolysis was complicated by acute renal failure. Labs at this initial presentation revealed peak creatinine kinase (CK) levels of 1,143,740 units/L (reference range: 3–300 U/L) and elevated lactic acid with a peak of 20 mmol/L (ref range: 0.7–2.1). Other biochemical abnormalities included multiple elevations in acylcarnitine profile including acetylcarnitine (C2), butyrylcarnitine (C4OH) and 3-hydroxyisovalerylcarnitine (C5-OH) (Table S1). Free carnitine was low at 11 μmol/L (ref range: 22–63) with total carnitine of 69 μmol/L (31–78). Plasma amino acids and urine acylglycines were normal. Urine organic acids showed multiple elevations (Table S1). He was in the hospital for a month and received hemodialysis, high dextrose-containing intravenous fluids, and intravenous levocarnitine during this initial episode.
Although this was the patient's first confirmed episode of rhabdomyolysis, the patient had a longstanding history of muscle weakness and exercise intolerance. His mother described him as a child who was not physically active and always wanted to be carried. There was no reported history of postnatal growth deficiency or developmental delays. His weight was 51.5 kg (96 percentile), height 152.4 cm (92 percentile) at age 10 years old. He currently weighs 129.5 kg and is 188.5 cm tall. The patient is of mixed European and Native American ancestry. His parents are distantly consanguineous with the patient's mother and father sharing a great grandparent (great-great-grandparent of the patient). The patient's family history is otherwise noncontributory. However, both parents reported a history of easy fatigability. A biceps muscle biopsy performed after the initial episode of rhabdomyolysis revealed evidence of chronic myopathy, fiber type II atrophy, and mitochondropathy. Electron microscopy showed increased non-membrane bound glycogen, fibrosis, and mitochondria of varying sizes with abnormal cristae and paracrystalline arrays. Histochemical testing for myophosphorylase, phosphofructokinase, lactate dehydrogenase, and myoadenylate deaminase was normal. Mitochondrial DNA content (qPCR) analysis, tissue specific mitochondrial genome sequencing and analysis of mitochondrial subunit assembly into respiratory chain complexes using blue native polyacrylamide gel electrophoresis were not done. The patient's clinical phenotype was attributed to mitochondrial myopathy, and he was started on home regimen of levocarnitine, leucovorin, CoQ10, B-complex vitamins, alpha-lipoic acid, and creatine. He had suboptimal compliance with this regimen.
Since his initial severe episode of metabolic decompensation, the patient has experienced 14 episodes of acute rhabdomyolysis (CK ranging from 900 to 60,206 U/L) with lactic acidosis (3.3–12.1 mmol/L). Apparent triggers for these episodes have included infection and even minor physical exertion such as walking around his home. Other than during the initial episode at the age of 10 years, he has not developed acute kidney injury, possibly related to increased monitoring and hydration support since this initial episode. During a recent acute rhabdomyolysis episode, elevation of branched-chain amino acids with altered ratios with elevation of alloisoleucine were noted in plasma amino acids (Table S1), although it should be noted this was in the setting of total parenteral nutrition. Acylcarnitine analysis showed elevation of C2, C5OH, and C16:1 (Table S1). Outside of these episodes, his baseline CK and lactates have been normal. Recent baseline plasma growth differentiation factor 15 (GDF15) was elevated at 1713 pg/ml (≤750) providing further supportive evidence of mitochondrial myopathy. The patient has required physical therapy after episodes of prolonged hospitalization due to rhabdomyolysis. He has easy fatiguability at baseline. He describes significant motor challenges. He has difficulty walking around in the house and taking a shower. He uses a wheelchair to cover short distances. His recent neurology examination revealed normal muscle bulk and tone with 2+ reflexes. He has no symptoms suggestive of a peripheral neuropathy, though a nerve conduction study has not been performed and there are no hearing concerns. Echocardiogram, ophthalmology evaluation and brain MRI/MRS completed with a recent hospitalization were normal. Renal (eGFR by Cystatin C = 123 (≥60 ml/min/BSA) and liver function tests were also normal.
The patient's emergency treatment for an episode of acute metabolic decompensation includes dextrose-containing intravenous fluids. We noted worsening lactic acidosis with high glucose infusion during a recent episode of rhabdomyolysis. A 75 g oral glucose load during the recovery phase of this admission resulted in a 1.5 mM (>2-fold) increase in lactic acid. Very high dextrose containing fluids have since been avoided due to this mitochondrial disorder. Additional calories have been supplemented using intralipid if needed. We have also recommended he avoids excess carbohydrate intake in his diet due to the noted carbohydrate sensitivity and obesity.
2.1 Genetic testing and untargeted global metabolomics results
Genetic testing was undertaken to identify the underlying molecular basis for the patient's symptoms. Initially, this included CPT2 sequencing and del/dup, LPIN1 sequencing and LPIN1 exon 18–19 deletion test. This identified a single heterozygous variant of uncertain significance (VUS) in CPT2: c.353A>G (p.D118G). Since his acylcarnitine analysis did not show a profile consistent with CPT2 deficiency, this variant was thought to be of no specific clinical significance. Mitochondrial DNA genome sequencing and deletion analysis plus 3243 A>G MELAS mutation testing was performed on skeletal muscle and revealed three sequence VUS. Subsequently, myopathy/rhabdomyolysis panel by massively parallel sequencing revealed an apparently homozygous VUS in ACADS (c.625G>A) and heterozygous VUS in CPT2 (c.353A>G) and POLG2 (c.1247G>C). POLG2 as well as CPT2 deletion/duplication analysis was negative.
At the age of 12 years, duo exome sequencing (ES) was performed including the proband and his mother. This was interpreted as negative with no pathogenic variants or variants of potential interest identified beyond the previously identified single variant in CPT2. Approximately three years later re-analysis of the ES data was requested. This identified a homozygous likely pathogenic variant in FDX2 (also known as FDX1L), NM_001031734.3: c.12G>T (p.M4I), NC_000019.9 (GRCh37 chr19):g.10426670 C>A. The patient's mother was found to be heterozygous for this variant. The father was not available for testing, but the variant was most likely inherited from each parent given consanguinity. This variant was initially described as c.3G>T (p.M1?) based on a previous version of the RefSeq transcript NM_001031734.2. This heterozygous missense change is ultra-rare in population databases (seen in 3 out of 265,278 alleles in gnomAD). The altered amino acid is conserved throughout evolution with high constraint at this position (score 4.96 in GERP). The methionine at position 4 of FDX2 is in the protein's highly conserved mitochondrial targeting sequence and it is possible that an alteration of this methionine to isoleucine at position 4 might disrupt its targeting to mitochondria and/or its protein stability. This alteration is predicted to be probably damaging (score 0.954) by PolyPhen in silico analysis, and predicted as deleterious by MutationTaster and DANN, with CADD score 24.8.
Untargeted global metabolomics testing was completed on a blood sample to better understand the biochemical perturbations in this rare disorder. Significant elevations of branched-chain keto-acids and hydroxyacids with normal branched-chain amino acids levels were noted. Elevations in mitochondrial intermediates such as lactate, 3-methylglutaconate, ethylmalonate, and 2-hydroxyglutarate indicative of mitochondrial dysfunction were found (Table S1).
3 DISCUSSION
Rhabdomyolysis is characterized by skeletal muscle cellular injury resulting in the release of intracellular components into the bloodstream resulting in elevated CK, and myoglobinuria that can precipitate acute renal failure. Recurrent rhabdomyolysis with exercise intolerance in an individual raises suspicion for an underlying genetic etiology. Common metabolic causes include fatty acid oxidation disorders (FAOD), lipin deficiency, glycogen storage disorders (GSD) and mitochondrial myopathies. Muscle biopsy can help in the differentiation of etiologies. Unlike FAOD and GSD, a specific trigger may not be identifiable in mitochondrial myopathies, though physical activity and other illnesses have been found to precipitate some of these episodes (Nance & Mammen, 2015; Scalco et al., 2015). Marked elevation in CK is not a classic feature of mitochondrial myopathies apart from TK2-related myopathic phenotype (Parikh et al., 2017).
Lebigot et al. and Spiegel et al. have described two individuals with progressive myopathy, lactic acidemia, and recurrent episodes of rhabdomyolysis (Lebigot et al., 2017; Spiegel et al., 2014). Both patients had a homozygous variant in FDX2 very similar to that found in the currently reported patient. Reported in these other patients initially as c.1A>T (p.M1?) based on transcript NM001031734.2, this variant corresponds to c.10A>T (p.M4L) in transcript NM_001031734.3, and hence, is in the same codon as the currently reported patient's c.12G>T (p.M4I). The clinical features of the currently described patient are similar (but more severe) to those described by these authors (Table 1). In terms of biochemical characterization, Spiegel et al. demonstrated that the M4L (previously called M1?) change results in nearly undetectable levels of FDX2 protein in isolated mitochondria from muscle or fibroblasts. While Spiegel et al. had explained the lack of detectable protein in mitochondria as due to translation start site alteration based on the earlier reference transcript, alteration of the mitochondrial localization sequence can also explain this absence even with the start at an upstream ATG in the newer reference. In terms of impact on downstream enzyme activities, Spiegel et al. reported decreased activity of complexes I, II, and III of the respiratory chain and reduced aconitase activity. Lebigot et al. found reduced complex I activity and reduced PDH complex activity, but normal complexes II and III, Aconitase activity and αKGDH activity. Additional evaluations of a muscle biopsy specimen including mitochondrial DNA content (qPCR) analysis, tissue specific mitochondrial genome sequencing and analysis of mitochondrial subunit assembly into respiratory chain complexes using blue native polyacrylamide gel electrophoresis were not done on these patients or our patient. Despite the variability in these reports suggesting a need for further characterization both reports nonetheless highlight the impact of FDX2 deficiency in mitochondrial function.
| Spiegel et al. (2014) | Lebigot et al. (2017) | Gurgel-Giannetti et al. (2018) | Currently reported patient; 2021 | |
|---|---|---|---|---|
| Age at initial presentation | 12 years | 5 months | 6 months −1.3 years | 10 years |
| Sex | Female | Female | ND | Male |
| Number of individuals | 1 | 1 | 6 | 1 |
Genotype (FDX2 variant) |
NM_001031734.3 Homozygous c.10A>T (p.M4L)a |
NM_001031734.3 Homozygous c.10A>T (p.M4L)a |
NM_001031734.3 Homozygous c.431C>T (p.P144L) |
NM_001031734.3 Homozygous c.12G>T (p.M4I)b |
| Progressive myopathy | + | + | + | + |
| Exercise induced myalgia | + | ND | + | + |
| Acute rhabdomyolysis | + | + | + (1/6 patients) | + |
| Optic atrophy | ND | ND | + | − |
| Abnormal brain MRI | ND | − | + | − |
| Hematologic abnormalities (microcytic anemia and neutropenia) | ND | ND | + (4/6 patients) | − |
| Plasma lactate, mmol/L | 7.8 | 23 | ND | 20 |
| Peak CK, U/L | 31,000 | ND | 3000 (1/6 subject) | >1,000,000 |
| Mitochondrial respiratory chain analysis | Reduced complexes I, II, and III activity (muscle and fibroblast) |
Reduced complex 1 activity (muscle) |
Normal (1/6 subjects) (muscle) |
Not done |
| Aconitase/CS activity | Reduced | Normal | ND | Not done |
| Muscle biopsy | Normal | ND | SDH- COX- fibers | Fiber type II atrophy, increased non membrane bound glycogen, abnormal mitochondria and fibrosis |
| Urine organic acid | Elevated 3-methyl glutaconic acid | ND | ND | Elevated lactic acid, pyruvic, acetoacetic acid and 3-hydroxy butyric acid |
| Plasma Amino acids | ND | ND | ND | Intermittent elevation of isoleucine, valine, leucine and alloisoleucine |
| Medications | Coenzyme Q10, riboflavin and thiamin | ND | ND | Coenzyme Q10, alpha lipoic acid, leucovorin, levocarnitine, MCT oil, B-complex vitamins |
- Abbreviation: ND, not documented.
- a The variants reported by Spiegel et al. in 2014 and Lebigot et al. in 2017 were originally reported as affecting in p.Met1? based on the RefSeq transcript NM_001031734.2 but would be p.Met4L based on the current RefSeq transcript NM_001031734.3.
- b Note that this alteration was previously described in currently reported patient as c.3G>T (p.M1?) based on the previous version of RefSeq transcript NM_001031734.2.
In 2018, Gurgel-Giannetti et al. expanded the phenotype associated with FDX2-related disorder (Gurgel-Giannetti et al., 2018). They described patients with optic atrophy, sensory motor axonal neuropathy leading to progressive myopathy, and recurrent episodes of cramps and myalgia in the first or second decade of life. The most common brain anomalies in this group of patients included hypoplastic optic nerves and chiasm. Three individuals among the described families also presented with T2 hyperintensity involving subcortical and deep cerebral white matter, corpus callosum, anterior thalamic nuclei and pons associated with restricted diffusion that became more evident between 3 and 5 years of age. All three of these patients had remarkable improvement that was noticeable in a follow-up MRI by the age of 7–9 years of life. Two patients from this group who had brain imaging during their adulthood did not show any abnormality. Brain MRI in the patient reported by Lebigot et al. was normal (Lebigot et al., 2017). Ophthalmology evaluation and brain MRI did not detect any abnormalities in the currently reported patient. All six patients reported by Gurgel-Gianetti et al. had a homozygous missense change (p.P144L) in a different part of the FDX2 protein from that in our patient or those reported by Spiegel et al. and Lebigot et al., impacting the metal ion binding region instead of the mitochondrial localization sequence. Respiratory chain analysis performed only on one patient in this case series was normal.
Clinical biochemical abnormalities outside of elevated CK, myoglobinuria and lactic acidosis are not well documented in this disease to date. Untargeted metabolomics in FDX2-related mitochondrial myopathy have not been described before. Untargeted metabolomics has been previously described as an effective tool in analyzing potential biomarkers and understanding the biochemical mechanisms associated with many metabolic disorders (Johnson et al., 2016; Miller et al., 2015). Results on a plasma sample obtained during one of the severe metabolic crisis episodes in this patient revealed elevations of branched-chain keto acid metabolites and alteration of ratios of the branched chain amino acids to each other suggestive of reduced BCKDH activity (Table S1). Plasma amino acids also showed mild elevation of the branched-chain amino acids at the time of decompensation. Confusing the picture, the patient was on total parenteral nutrition (TPN) at the time of blood draw for plasma amino acids. However, the presence of diagnostic level of alloisoleucine in plasma amino acids along with multiple keto-acid metabolites detected by untargeted metabolomics obtained before the initiation of TPN supports the hypothesis that activity of BCKDH is affected in this disorder due to deficient lipoate cofactor biosynthesis.
Global metabolomic profile also showed multiple elevations of mitochondrial intermediates including lactate, 3-methylglutaconate, ethylmalonate, and 2-hydroxyglutarate, suggestive of α–KGDH deficiency (Table S1). Fe–S proteins also play an important role in the functioning of biotin synthase (Figure 1) (Broach & Jarrett, 2006; Reyda et al., 2009). Elevation in 3-hydroxyisovalerylcarnitine (C5-OH) detected in plasma Acylcarnitine profile along with untargeted global metabolomics may be explained by the deficiency of biotin synthase. Biotin supplementation thus could be of benefit in patients with FDX2-related disorder. Further research is needed to confirm these findings.
Inadequate aerobic respiration and decreased functioning of tricarboxylic acid (TCA) cycle in PDH deficiency can biochemically present with worsening of lactic acidosis with high dextrose administration. During a recent hospital admission for rhabdomyolysis, the currently reported patient was noted to have sensitivity to high dextrose-containing intravenous fluids evidenced by rising lactate and, possibly, CK. An oral glucose load also led to increased lactate levels. Intravenous hydration with dextrose-free fluids and caloric supplementation with intralipid infusion correlated with a downward trend of CK and lactate to normal limits. Complicating this picture on a subsequent admission, the effect of dextrose infusion was more muted. We speculate that this change may reflect increased lipoate availability in the setting of interval increased alpha-lipoic acid supplementation. None of the other previously published articles have commented on the possibility of ketogenic diet in this disorder. Avoidance of excess carbohydrate intake has been recommended to our patient due to the noted carbohydrate sensitivity and obesity.
Multiple vitamins and cofactors are employed in the treatment of mitochondrial disease, even though such therapies are not standardized and there are numerous variations of treatment (Lehmann & McFarland, 2018; Parikh et al., 2015). This patient was started on coenzyme Q10, alpha-lipoic acid, MCT oil, B-complex vitamins and levocarnitine. The dose of alpha-lipoic acid was maximized after the recent biochemical abnormalities were detected. Further studies and long-term follow-up of this patient are required to improve understanding of the perturbed biochemical pathways in this disorder.
Our report highlights the importance of considering FDX2 deficiency in individuals presenting with recurrent rhabdomyolysis. In addition, based on this patient's experience, we recommend dextrose should be provided only in limited quantity or carefully monitored for effect on lactate levels during episodes of rhabdomyolysis to avoid worsening lactic acidosis. This case adds to the growing body of literature demonstrating the potential for ES re-analysis to identify diagnostic variants in suspected cases that remain undiagnosed after initial ES (Fung et al., 2020; Salfati et al., 2019).
ACKNOWLEDGMENTS
The authors would like to thank the patient, his family and all physicians involved in his care especially Dr. Timothy J. Feyma.
Patient gave written informed consent for publication.
CONFLICT OF INTEREST
Authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Anjali Aggarwal: Conceptualization, Writing—Original Draft, Visualization, Writing—Review & Editing. Nishitha R. Pillai: Conceptualization, Writing—Original Draft, Visualization, Writing - Review & Editing. Charles J. Billington Jr.: Writing—Review & Editing. Lynn Schema: Writing—Original Draft, Writing—Review & Editing. Susan A. Berry: Writing—Review & Editing.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.