Improving survival in patients with trisomy 18
Shoko Tamaki and Sota Iwatani contributed equally to this study.
Abstract
The effects of medical and surgical interventions on the survival of patients with trisomy 18 have been reported, leading to changes in perinatal management and decision-making. However, few studies have fully reported the recent changes in survival and treatment of trisomy 18. We examined how treatment and survival of patients with trisomy 18 have changed over a decade in a Japanese pediatric tertiary referral center. This retrospective cohort study included patients with trisomy 18 who were admitted within the first 7 days of life at the Hyogo Prefectural Kobe Children's Hospital between 2008 and 2017. The patients were divided into early period (EP) and late period (LP) groups based on the birth year of 2008–2012 and 2013–2017, respectively. Changes in treatment and survival rates were compared between the two groups. A total of 56 patients were studied (29 in the EP group and 27 in the LP group). One-year survival rates were 34.5% and 59.3% in the EP and LP groups, respectively. The survival to discharge rate significantly increased from 27.6% in the EP group to 81.5% in the LP group (p < 0.001). The proportion of patients receiving surgery, especially for congenital heart defects, significantly increased from 59% in the EP group to 96% in the LP group (p = 0.001). In our single-center study, survival and survival to discharge were significantly improved in patients with trisomy 18, probably because of increased rate of surgical interventions. These findings may facilitate better decision-making by patients' families and healthcare providers.
1 INTRODUCTION
Trisomy 18 was first described in 1960 by Edwards et al. It is the second most common autosomal trisomy in newborns, with an estimated incidence of 1/6000 live births (Rasmussen et al., 2003; Root & Carey, 1994). The syndrome typically manifests as prenatal-onset growth deficiency and developmental disability, multiple major congenital anomalies in cardiovascular, respiratory, gastrointestinal, and skeletal, and tumor susceptibility (Cereda & Carey, 2012). Trisomy 18 has been previously referred to as a “lethal anomaly” due to high mortality rate and severe developmental disability among survivors (McGraw & Perlman, 2008). In fact, withholding or withdrawal of intensive treatment had been recommended for patients with a poor prognosis (Bos et al., 1992; McGraw & Perlman, 2008; Pyle et al., 2018).
Meanwhile, questionnaire-based natural history studies by Baty et al. (1994) and Kosho et al. (2013) demonstrated that a small proportion of children with trisomy 18 can survive for long periods and continue to learn motor and communication skills. Based on these findings, the medical approaches for trisomy 18 have been changing over the past two decades (Silberberg et al., 2020). In 2004, the Japanese guidelines proposed that a treatment decision should be in the “best interests of the child,” based on deep discussions with the patients' family (Japan Society for Neonatal Health and Development, 2004; Kosho, 2008). In 2011, Nelson et al. revealed that the number of hospitalizations for trisomy 18 significantly increased from 1036 to 1616 per year between 1997 and 2009 in the United States (Nelson et al., 2012). Around the same time, an increasing number of studies performed a variety of investigations that evaluated the effectiveness of intensive care and surgical interventions on the survival of patients with trisomy 18. These studies have focused on the effects of congenital heart defects (CHD) and esophageal atresia (EA) on survival (Graham et al., 2004; Iida et al., 2020; Kaneko et al., 2008; Kosho et al., 2006; Kosiv et al., 2017; Maeda et al., 2011; Nakai et al., 2016; Nishi et al., 2014; Peterson et al., 2017). Such recent changes in the management of patients with trisomy 18 should be shared with patients' families and health providers. However, some of these studies are small case series with relatively short follow-up (Graham et al., 2004; Iida et al., 2020; Kaneko et al., 2008; Kosho et al., 2006; Maeda et al., 2011; Nakai et al., 2016; Nishi et al., 2014), and others included only patients who lived longer with fewer comorbidities (Kosiv et al., 2017; Peterson et al., 2017). Thus, it is difficult to capture the “whole picture” of the recent changes in treatment and survival of patients with trisomy 18. Here, we conducted a retrospective cohort study in patients with trisomy 18 at a Japanese pediatric tertiary referral center and further investigated how medical treatment and survival changed over a decade.
2 MATERIALS AND METHODS
2.1 Study design and patients
This was a retrospective cohort study by medical record review that was conducted at the Hyogo Prefectural Kobe Children's Hospital (HPKCH), a pediatric tertiary referral center that serves the entire area of Kobe city. HPKCH provides specialized medical care for critically ill children with the full complement of surgical services, including cardiothoracic surgery and neurosurgery. The HPKCH neonatal intensive care unit (NICU) is a 21-bed unit, with approximately 500 admissions annually that provides a full range of care for infants with complex diseases. Except for stillbirth, newborns with surgical diseases and/or chromosomal disorders are basically transferred to our NICU regardless of their clinical condition. Treatment decisions are made by health providers in partnership with the patients' parents. The primary consideration when making decisions is whether the treatment is in the child's best interests. Therefore, the potential burdens and benefits of treatment are individually assessed in view of the severity of patients' conditions and parents' wishes (Japan Society for Neonatal Health and Development, 2004; Silberberg et al., 2020).
In this study, patients with trisomy 18 that were admitted to the NICU within the first 7 days of life between 2008 and 2017 were investigated. Diagnosis of trisomy 18 was based on chromosomal analysis of amniotic fluid and/or neonatal blood or postnatal clinical evaluations. The patients were followed-up until death or the end of the study (December 31, 2020), and those lost to follow-up were excluded from the study. To investigate the changes in medical treatments and survival over time, the study patients were divided into two groups: the early period (EP) group, which comprised patients who were born between 2008 and 2012 and the late period (LP) group, which comprised those born between 2013 and 2017.
2.2 Ethical statement
The requirement for informed consent was waived by the Institutional Review Board due to the retrospective design of the study. Instead, we informed the public about this study via the hospital bulletin board and website. The Institutional Review Board of Hyogo Prefectural Kobe Children's Hospital (R2-101) approved this study.
2.3 Medical treatment
Based on “the best interest of the child,” all medical treatments, including perinatal management and postnatal intensive care, were provided to each patient, with the consent of their family. According to the patients' condition, intubation at birth, ventilatory support, blood transfusion, blood pressure support, and parenteral nutrition were provided during intensive care. After they were discharged, emergency care providers and general pediatricians within the hospital addressed acute and chronic problems in children with special health care needs. Specific therapy, such as prophylactic antibiotics for preventing airway infection and pulmonary vasodilator for pulmonary hypertension (PH) related with CHD, was not routinely performed during the study period.
2.4 Surgical treatment
In addition to medical treatment, all surgeries were performed after discussion and agreement with the patients' families. Informed surgical decision-making and carefully weighing of potential benefits and risks of surgery were done. Surgical risks were individually assessed based on the patient's weight and clinical conditions. Since patients with trisomy 18 and EA often have low birth weight, our first approach for EA has been nonradical surgery, such as esophageal banding or tracheoesophageal fistula (TEF) division with a feeding gastrostomy (Hasebe et al., 2021). When the gap between the upper and lower esophageal segments was short, we performed total repair of the EA. For the same reason, patent ductus arteriosus (PDA) ligation and/or pulmonary artery banding (PAB), instead of radical surgery, was first proposed for cases of CHD with a left-to-right shunt.
2.5 Statistical analysis
Descriptive statistics were summarized using means (standard deviations), median (95% confidence interval [CI]), or counts (percentages), as appropriate. Group comparisons were performed using Fisher's exact test or Pearson's χ2 test for categorical variables, or the Wilcoxon rank-sum test for continuous variables. To compare survival, the overall survival for each group was estimated using the Kaplan–Meier method. Patients who were alive at the end of the study were censored. Statistical significance between survival curves was determined using the log-rank test. Statistical significance was set at p < 0.05. All analyses were performed using JMP statistical software (JMP 13.0.0; SAS Institute, Inc.).
3 RESULTS
3.1 Patient characteristics
Between 2008 and 2017, 60 patients with trisomy 18 were admitted to the NICU within the first 7 days of life. All patients were candidates for intensive care, including resuscitation at birth, endotracheal intubation, and ventilatory support. When the patients were likely to benefit from surgical interventions, the parents were fully informed about the surgical prognosis and complications for shared decision-making. Four patients (two in each group) who were lost to follow-up were excluded. A total of 56 patients (29 in the EP group and 27 in the LP group) were included in this analysis. The patient characteristics are shown in Table 1. No significant differences were observed between the two groups. The detailed clinical information of each patient is described in Table S1.
| All | EP group | LP group | p Value | |
|---|---|---|---|---|
| n = 56 | n = 29 | n = 27 | ||
| Gestational age (weeks) | 36.5 ± 3.1 | 35.8 ± 3.6 | 37.2 ± 2.5 | 0.123 |
| Birth weight (g) | 1603 ± 423 | 1520 ± 459 | 1694 ± 366 | 0.123 |
| Birth weight < 1500 g | 21 (38) | 13 (45) | 8 (30) | 0.280 |
| Female | 38 (68) | 17 (59) | 21 (78) | 0.158 |
| Cesarean delivery | 42 (75) | 20 (69) | 22 (81) | 0.361 |
| Apgar score < 7 at 5 min | 23 (41) | 15 (52) | 8 (30) | 0.111 |
| Cardiac anomalies | 55 (98) | 28 (97) | 27 (100) | 1.000 |
| PDA | 45 | 24 | 21 | |
| VSD/DORV-VSD | 42 | 23 | 19 | |
| TOF/DORV-TOF | 8 | 2 | 6 | |
| AVSD | 2 | 1 | 1 | |
| CoA/IAA | 7 | 2 | 5 | |
| HLHS | 2 | 1 | 1 | |
| Esophageal atresia | 16 (29) | 11 (38) | 5 (19) | 0.144 |
| Gross type C | 16 | 11 | 5 | |
| Meningomyelocele | 3 (5) | 2 (7) | 1 (4) | 1.000 |
- Note: Data are presented as the mean ± SD or n (%). Abbreviations: AVSD, atrioventricular septal defect; CoA, coarctation of the aorta; DORV, double-outlet right ventricle; EP, early period; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; LP, late period; PDA, patent ductus arteriosus; VSD, ventricular septal defect; TOF, tetralogy of Fallot.
3.2 Changes of medical treatment
Table S2 shows the medical treatments provided, including intensive care. Overall, the rate of intubation at birth was 41%, which was comparable between the two groups (EP group: 48% vs. LP group: 33%, p = 0.289). The rate of ventilatory support was 96%; it was also similar between the two groups (EP group: 100% vs. LP group: 93%, p = 0.228).
3.3 Changes of surgical treatment
Table 2 shows the surgical procedures performed for each group. A total of 43 patients (77%) received surgical treatment. The proportion of patients that received surgery increased from 59% in the EP group to 96% in the LP group (p = 0.001). The rate of EA surgery was not significantly different between the two groups (EP group: 73% vs. LP group: 100%, p = 0.509). Three of the five patients in the LP group underwent total repair of EA, whereas none of the patients in the EP group underwent the procedure. In particular, the rate of cardiac surgery increased significantly in the LP group (EP group: 36% vs. LP group: 67%, p = 0.032). Tracheostomy was performed in 19 patients (34%). The tracheostomy rate increased significantly in the LP group (EP group: 21% vs. LP group: 48%, p = 0.048).
| All | EP group | LP group | p Value | |
|---|---|---|---|---|
| n = 56 | n = 29 | n = 27 | ||
| Total surgery | 43/56 (77) | 17/29 (59) | 26/27 (96) | 0.001 |
| Esophageal atresia surgery | 13/16 (81) | 8/11 (73) | 5/5 (100) | 0.509 |
| Postnatal age at the first surgery (days) | 1 (0–10) | 2 (1–10) | 0 (0–1) | 0.004 |
| Gastrostomy | 12 | 8 | 4 | |
| Esophageal banding | 9 | 8 | 1 | |
| Tracheoesophageal fistula division | 4 | 3 | 1 | |
| Total repair | 3 | 0 | 3 | |
| Cardiac surgery | 28/55 (51) | 10/28 (36) | 18/27 (67) | 0.032 |
| Postnatal age at the first surgery (days) | 31 (3–163) | 45 (3–163) | 28 (7–98) | 0.164 |
| PDA closure | 21 | 7 | 14 | |
| Pulmonary artery banding | 25 | 8 | 17 | |
| VSD repair | 2 | 1 | 1 | |
| Tracheostomy | 19/56 (34) | 6/29 (21) | 13/27 (48) | 0.048 |
| Postnatal age at surgery (months) | 7.2 (2.6–137.4) | 6.8 (5.6–137.4) | 8.2 (2.6–83.6) | 0.726 |
- Note: Data are presented as the median (range) or n (%). Abbreviations: EP, early period; LP, late period; PDA, patent ductus arteriosus; VSD, ventricular septal defect.
The median postnatal age at the first surgery for EA was significantly earlier in the LP group (EP group: 2 days vs. LP group: 0 days, p = 0.004) than in the EP group. The first surgery for CHD was also done earlier in the LP group compared to when it was done in the EP group (EP group: 45 days vs. LP group: 28 days, p = 0.164), although the difference was not statistically significant.
3.4 Survival analysis
The Kaplan–Meier survival curves for each group are shown in Figure 1. The median survival in all the patients was 314 days (95% CI: 202–549) (Figure 1a). The median survival in the EP and LP groups was 180 days (95% CI: 56–453) and 535 days (95% CI: 299–1771), respectively (Figure 1b). The log-rank test demonstrated significantly improved survival in the LP group (p = 0.005, log-rank test).
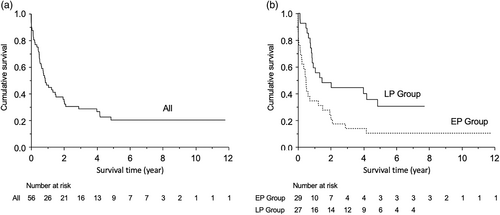
As shown in Table 3, the overall survival rates at 1-, 2-, and 3-year was 46.4%, 35.7%, and 28.6%, respectively. In comparison to the respective survival rates, those at 1 and 2 years were not significantly different, whereas at 3 years, survival rates were significantly higher in the LP group (EP group: 13.8% vs. LP group: 44.4%, p = 0.017).
| All | EP group | LP group | p Value | |
|---|---|---|---|---|
| n = 56 | n = 29 | n = 27 | ||
| 1-Year survival rate | 46.4 (33.9–59.4) | 34.5 (19.7–56.4) | 59.3 (40.3–75.8) | 0.107 |
| 2-Year survival rate | 35.7 (22.8–47.2) | 24.1 (12.0–42.7) | 48.2 (30.4–66.4) | 0.094 |
| 3-Year survival rate | 28.6 (18.3–41.7) | 13.8 (5.3–31.5) | 44.4 (27.2–63.1) | 0.017 |
- Note: Data are presented as % (95% confidence interval). Abbreviations: EP, early period; LP, late period.
3.5 Survival to discharge
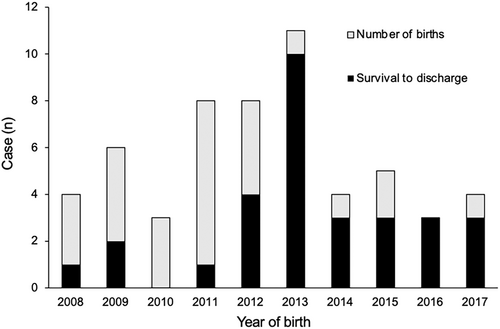
Thirty patients (52.6%) survived to discharge. Trends in survival to discharge by birth year are shown in Figure 2. The survival to discharge rate significantly increased from 27.6% (8/29) in the EP group to 81.5% (22/27) in the LP group (p < 0.001). However, medical therapies at discharge, including feeding and respiratory support, had not changed significantly between the two groups as shown in Table S3.
Among the 56 patients, 44 (78.6%) died during the study period (26 in the EP group and 18 in the LP group). The causes of death are summarized in Table 4. Chronic heart failure (CHF) was the leading cause of death in both groups. Among the 16 patients who died of CHF, 8 in the EP group and 3 in the LP group did not undergo cardiac surgery. The second leading cause of death was severe birth asphyxia in the EP group and infection in the LP group. Nine patients (three in the EP group and six in the LP group) died suddenly and unexpectedly at home (cause unknown).
| All | EP group | LP group | |
|---|---|---|---|
| n = 44 | n = 26 | n = 18 | |
| Chronic heart failure | 16 (36) | 11 (42) | 5 (28) |
| Severe birth asphyxia | 4 (9) | 4 (15) | 0 (0) |
| Ventricular arrhythmias | 3 (7) | 2 (8) | 1 (6) |
| Hepatoblastoma | 3 (7) | 2 (8) | 1 (6) |
| Liver failure | 2 (5) | 2 (8) | 0 (0) |
| Infection | 4 (9) | 1 (4) | 3 (17) |
| Gastric perforation | 1 (2) | 1 (4) | 0 (0) |
| Seizure | 1 (2) | 0 (0) | 1 (6) |
| Chylothorax | 1 (2) | 0 (0) | 1 (6) |
| Sudden death, cause unknown | 9 (20) | 3 (12) | 6 (33) |
- Note: Data are presented as n (%). Abbreviations: EP, early period; LP, late period.
4 DISCUSSION
In this retrospective cohort study at a Japanese pediatric tertiary referral center, the 1-year survival rate in patients with trisomy 18 increased from 34.5% during 2008–2012 (EP group) to 59.3% during 2013–2017 (LP group) (Table 3). Population-based studies of survival among patients with trisomy 18 in the United States (1999–2007) (Meyer et al., 2016) and Canada (1991–2012) (Nelson et al., 2016) reported 1-year survival rates of 13.4% and 12.6%, respectively. In Japan, a multicenter study at nine institutions affiliated with Nagoya University showed a 1-year survival rate of 29.1% among 117 newborn patients with trisomy 18 who were admitted between 2000 and 2015 (Kato et al., 2019). Compared to these prior reports, the 1-year survival rate shown in our cohort was relatively higher and increased over time. Moreover, survival rates in the LP group were still high, even at 2 and 3 years of age (48.2% and 44.4%, respectively). Although these results were obtained from a single institution study, we have shown markedly improved survival in patients with trisomy 18 in recent years.
Improved survival among patients with trisomy 18 in our study could be explained by the recent changes in surgical treatments, and this is consistent with the findings of other studies on the effects of surgeries (Graham et al., 2004; Iida et al., 2020; Kaneko et al., 2008; Kosho et al., 2006; Kosiv et al., 2017; Maeda et al., 2011; Nakai et al., 2016; Nishi et al., 2014; Peterson et al., 2017). In comparison to medical treatments in our cohort, a comparable proportion of patients in both the EP and the LP groups received intensive care, such as intubation at birth and ventilatory support. This indicates that no significant changes occurred in the initial medical management of patients with trisomy 18. In contrast, the proportion of patients who underwent surgeries significantly increased from 59% in the EP group to 96% in the LP group (Table 2). Furthermore, the postnatal ages at the first surgery were earlier in the LP group, especially for EA and CHD. An increase in the number of patients who received surgical treatment at an earlier age likely contributed to the improvement in survival over time. We speculate that this change was based on the accumulated surgical experience at our institution. Specifically, EA and CHD in trisomy 18 became gradually known not only to neonatologists, but also to surgeons and anesthesiologists. Wider recognition and better understanding of these surgical diseases might have enabled earlier decisions of surgical treatments, further led to improvement in survival.
In our study, the proportion of patients with trisomy 18 who underwent EA surgery did not significantly change over time. However, the median postnatal age during the first surgery for EA was significantly lower in the LP group (EP group: 2 days vs. LP group: 0 days), and this may have contributed to improved survival. Particularly in cases of EA with TEF, earlier surgery is potentially beneficial in order to prevent complications, such as gastric distension, subsequent gastric perforation, and respiratory failure due to leakage of the ventilatory gases through the TEF (Filston et al., 1984).
Our study clearly demonstrated that the proportion of patients with trisomy 18 who underwent cardiac surgery significantly increased from 36% to 67% over time. Furthermore, the median age at the first surgery for CHD was earlier in the LP group (EP group: 45 days vs. LP group: 28 days), as well as surgery for EA, although the difference was not statistically significant. In 2017, Peterson et al. (2017) reported that patients with trisomy 18 who died in-hospital following cardiac surgery had a high mean pulmonary artery pressure and pulmonary vascular resistance during preoperative cardiac catheterization. This indicates that cardiac surgery before the development of PH may decrease postoperative mortality in patients with trisomy 18. Recently, Tahara et al. (2021) described the histological findings of the lung tissue at the first cardiac surgery (mean age of 37 days) in patients with trisomy 18, indicating the possibility of irreversible pulmonary vascular changes due to PH, such as intimal fibrous proliferation, at an earlier age. Moreover, they suggested that abnormal lung development (medial defects and/or hypoplasia of the small pulmonary artery, alveolar hypoplasia) in patients with trisomy 18 may be associated with the earlier development of PH. Considering these findings, performing surgery earlier in those with CHD in the LP group might have contributed to improved survival.
So far, there are only a limited number of studies on the survival to discharge rate in patients with trisomy 18, and this ranges from 21% to 55% (Iida et al., 2020; Kosho et al., 2006; Nakai et al., 2016). Our study showed an overall survival to discharge rate of 52.6%. Notably, the rate significantly increased from 27.6% in the EP group to 81.5% in the LP group. Information about the increasing probability of discharging a patient home is important to parents. A questionnaire study by Janvier et al. (2016) showed that more than half of the parents of patients with trisomy 18 hoped to bring their child home and be a family. Meanwhile, they also hoped that their child would not suffer and be harmed by interventions. Our findings of increased survival to discharge rate could also facilitate better decision-making between parents and health providers.
Most children with trisomy 18 have complex chronic conditions. They are often discharged from the NICU with medical devices, including tracheostomy, home mechanical ventilation, and tube feeding. These special health care needs lead to an increased risk of emergency department visits and readmission. Therefore, care coordination should be established among the emergency department, primary care, and multiple subspecialty providers, and this will contribute to improved survival to discharge.
In our cohort, CHF remained the leading cause of death during the study period, which is in agreement with the results of other studies (Cereda & Carey, 2012; Iida et al., 2020). The CHF should be related to the progression of PH, although not confirmed with echocardiography and/or catheterization study. Palliative surgery for CHD has been reported to be effective in preventing the development of heart failure and pulmonary hypertension even in patients with trisomy 18 (Graham et al., 2004; Kaneko et al., 2008; Kosiv et al., 2017; Maeda et al., 2011; Nakai et al., 2016). Although these reports showed improved short-term survival rates, the long-term effects on survival have been controversial. In contrast, a recent case series demonstrated longer survival (> 5 years) in patients with CHD that underwent complete repair (Nakai et al., 2021; Peterson et al., 2017), suggesting that complete repair is preferable to palliative surgery for selected patients with greater weight and simple cardiac defects. Only palliative surgery was performed in most of our patients, while complete repair of ventricular septal defect was performed in only two cases. We believe that palliative surgery, such as PDA ligation and/or PAB, is insufficient to reduce long-term mortality due to CHD.
This study has some limitations. First, our findings cannot be generalized to other NICU settings because this was a single-center study. However, it allowed the evaluation of not only the overall survival but also the changes in medical and surgical treatments in detail at a single pediatric tertiary referral center. Second, due to the retrospective study design, we cannot conclusively demonstrate the relationship between the observed improved survival and changes in treatments in our cohort. Unmeasured confounding factors may underlie the observed differences between the two groups. Finally, family background, such as parental age, socioeconomic status, and family structure, were not examined in this study, and these may have influenced the survival to discharge rate and home medical care.
5 CONCLUSION
In our retrospective cohort study, the 1-year survival rate of patients with trisomy 18 markedly increased from 34.5% during 2008–2012 to 59.3% during 2013–2017. In addition, the survival to discharge rate also significantly increased from 27.6% to 81.5%. An increase in the number of patients receiving surgical treatments for EA and CHD at an earlier age may have contributed to improved survival in our cohort. The study's findings are important and may facilitate decision-making between patients' families and healthcare providers.
ACKNOWLEDGMENTS
The authors thank all medical staff members who provided support to the patients and their families. Authors express particular gratitude to Drs. Eiji Nishijima, Kosaku Maeda, and Tadashi Hatakeyama at the Department of Pediatric Surgery; Tatsuya Nagashima and Atsushi Kawamura at the Department of Pediatric Neurosurgery; Toshikatsu Tanaka at the Department of Pediatric Cardiology, and Yoshihiro Ohshima at the Department of Pediatric Cardiovascular Surgery for surgical treatment.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualized the research project and drafted the initial manuscript: Shoko Tamaki and Sota Iwatani. Contributed to the acquisition of the clinical data: Ayako Izumi, Kentaro Hirayama, Dai Kataoka, Shohei Ohyama, Toshihiko Ikuta, Emiko Takeoka, Sachiko Matsui, Hitomi Mimura, Shogo Minamikawa, and Yasuo Nakagishi. Coordinated and supervised data collection, and critically reviewed the manuscript: Seiji Yoshimoto and Hideto Nakao.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.