Dysmorphism and major anomalies are a main predictor of survival in newborns admitted to the neonatal intensive care unit in the Democratic Republic of Congo
Funding information: Chair for Human Genetics in DR Congo; Marc Vervenne Fund Doctoral Scholarship
Abstract
In Central-Africa, neonatal infections, asphyxia and prematurity are main reasons for admission to the neonatal intensive care unit and major determinants of newborn survival. Also, the outcome of newborns with congenital anomalies is expected to be poor, due to a lack of state-of-the art care. We conducted a study of 102 newborns recruited in the Neonatal Intensive Care Unit (NICU) at the University Hospitals of Kinshasa, DR Congo, to assess the impact of congenital anomalies. The presence of a major anomaly was associated with a hazard ratio of death of 13.2 (95%CI: 3.7–46.7, p < .001). In addition, the presence of three or more minor anomalies was associated with a 4.5-fold increased risk of death (95%CI: 1.1–18.6, p = .04). We conclude that like major anomalies, the presence of three or more minor anomalies should also be given particular attention and that the evaluation of dysmorphism should be promoted in NICU.
1 INTRODUCTION
In Central Africa, an estimated one in seven children dies before the age of 5 years (Forae, Uchendu, & Igbe, 2014; Fotso, Ezeh, Madise, & Ciera, 2007). For children with a congenital anomaly, life expectancy is even lower (Modell et al., 2012). Reliable epidemiological data on the incidence of congenital malformations are scarce, despite the presence of risk factors such as the lack of screening programs, exposure to chemical and infectious agents, malnutrition, and also high rates of consanguinity in certain regions, advanced parental age, and large family size (Christianson, Howson, & Modell, 2006; WHO, 2011). To address this question, the World Health Organization (WHO) has emphasized the need to conduct epidemiological studies and needs assessments in low- and middle-income countries to determine the most prevalent congenital and genetic disorders and the burden they impose on health (WHO, 2011).
In low-income countries such as the Democratic Republic of Congo (DRC), the care for children with congenital anomalies currently has a low priority, mainly due to a poor prognosis and high cost of treatment, if available at all. In the Neonatal Intensive Care Unit (NICU) of the University Hospital of Kinshasa, congenital malformations are the fourth most frequent cause of mortality, after infections, neonatal asphyxia and jaundice (Biselele et al., 2013). However, access to treatment for congenital anomalies such as cleft lip/palate, club feet, and congenital heart disease is becoming increasingly available, either through local specialized physicians or through international charity organizations. These interventions improve survival and will thus increase the prevalence of such malformations in the population. At the same time, improved living conditions is being achieved by reducing the burden of environmental factors such as malnutrition and infectious diseases. Therefore, the relative contribution of congenital malformations to child mortality and morbidity will increase in comparison to other causes. This is in line with recent findings by Liu et al. (2015), who showed that decreased child mortality is mainly due to a reduction in infectious disease, whereas the decrease of the contribution of congenital malformations is limited. This transition is well illustrated in Europe, where congenital malformations account now for 25% of neonatal death, compared to 8% worldwide (WHO, 2004).
This increased importance of congenital malformations on neonatal mortality in low- and middle-income countries underscores the need to build up expertise in the evaluation of dysmorphological features in newborns and in the management of major congenital anomalies. In a previous study of unselected newborns born in two maternities in Kinshasa, DR Congo, we detected a prevalence of major anomalies of at least 2.2% and dysmorphism in 4.3% (Mubungu et al., 2020). However, we were not able to evaluate the known association of multiple minor anomalies with an increased risk of a major malformation (Hennekam, 2011) nor their contribution to the mortality. Further insight in the association between major and minor anomalies and their impact on mortality would, however, provide valuable information for the clinical assessment of newborns in Central African countries. We therefore conducted a prospective study in a cohort of newborns admitted to a single academic NICU in Kinshasa, DRC, a population in which we expected a higher prevalence of both minor anomalies and major malformations.
2 MATERIALS AND METHODS
2.1 Patients' selection and recruitment
This study was conducted in the Neonatal Intensive Care Unit (NICU), at the University Hospitals of Kinshasa, DR Congo. This is a tertiary institution that provides care to ill newborns from across the city.
All (n = 102) newborns admitted to the NICU between April 2017 and July 2019 were eligible for inclusion, but actual recruitment depended on the presence of the principal investigator (GM) at the time of admission. Parents were informed about the aims and design of the study and provided a signed informed consent. None of the parents declined to participate. The study was approved by the Ethical Committee of the Public Health School of the University of Kinshasa (ESP/CE/015/2018).
2.2 Data acquisition
All children were examined by the same clinician (GM). This included biometry, neurological examination, evaluation of dysmorphism and clinical photography, as described previously (Mubungu et al., 2020). A congenital physical anomaly was defined as an anatomic phenotype that deviates substantially from an appropriate reference population (Hennekam et al., 2013). Major anomalies were defined as congenital anomalies that have a significant impact on the health of the child, whereas minor anomalies, are those with little or no functional or esthetic impact. Morphological features that occur in 4% or more of children in a population are considered as common variants, while those that occur less frequently are considered dysmorphic or minor anomalies.
Morphologic features were defined according to the Elements of Dysmorphology (Allanson et al., 2009) and designated using the Human Phenotype Ontology (HPO) nomenclature. Additional information was extracted from the medical records. This included the reason(s) for admission and gestational age. Reasons for admission were classified as: prematurity, infection, asphyxia, or major malformation. Gestational age was determined using the Finnström score (Finnstrom, 1971). Children born between 37 and 42 weeks of amenorrhea were considered term, whereas those born before 37 weeks or after 42 weeks were preterm or after postterm, respectively.
Weight, length, and head circumference at birth were converted to z-scores using the reference curves for size at birth of the INTERGROWTH-21st PROJECT (Villar et al., 2014). Small for gestational age was defined as birth weight below the percentile 10, adjusted for gestational age.
Follow-up data on mortality of the newborns after discharge from the NICU were collected from the parents by phone 1 month, 4 months, 6 months, and 1 year after discharge.
2.2.1 Data management and statistical analysis
Data were recorded in an excel file and further analyzed with IBM SPSS Statistics version 22.0 and R version 4.0 (R foundation for Statistical Computing, Vienna, Austria). Continuous variables were summarized as means and standard deviations, and categorical variables as proportions with exact binomial 95% confidence intervals. The presence of dysmorphism according to child and parental characteristics, mortality according to premature birth, and the proportion of newborns with a major anomaly or other condition that impacts health according to the number of minor malformations was estimated with logistic regression. Results are expressed as odds ratio's (OR) with a 95% confidence interval. Survival up to 1 year after discharge of newborns admitted to the NICU was estimated with Kaplan–Meier curves. Survival according to the presence of a major malformation or minor anomalies was compared with a log-rank test, and Cox regression was used to estimate the hazard ratio in children with versus without anomalies adjusted for birth weight and gestational age. A p value <.05 was considered as statistically significant.
3 RESULTS
3.1 General characteristics
Over a period of 2 years, a total of 102 newborns were recruited in the NICU. Sixty newborns were male (60%) and 40 females (40%) and a conjoined pair of twins presented ambiguous external genitalia.
The newborns were admitted for infections (n = 82, 80.4%), prematurity (n = 29, 28.4%), asphyxia (n = 23, 22.5%), hemolytic disease (n = 19; 18.8%), congenital anomalies (n = 16, 15.7%), intraventricular hemorrhage (n = 13, 12.7%), or other (n = 32, 31.4%). There was more than one reason for admission for 77 (75.5%) children in the study. As for the mothers, there were 43 primipara, 56 multipara, and three with unknown parity. Eighty-five newborns were singletons, 17 multiples (eight twin pairs and one single child part of a twin). The mean gestational age was 37.3 ± 2.8 weeks (n = 86), and 41 out of 102 children were born premature and two postterm. The mean z-score of weight at birth was −0.71 ± 1.24 (n = 85) and 28 newborns (32.9%) were small for gestational age.
3.2 Minor anomalies
Table 1 presents the spectrum and prevalence of 62 different minor variants and anomalies that were observed in this study, along with their prevalence in a reference population of unselected newborns in Kinshasa (Mubungu et al., 2020). In total, 13 morphological anomalies were observed in the NICU but not in the reference population. Three of these did not have a HPO reference (Table 1).
| Common variants (≥4% in the population)a | Dysmorphism (<4% in the population)a | ||||||
|---|---|---|---|---|---|---|---|
| HPO | Phenotype | REF | NICU | HPO | Phenotype | REF | NICU |
| HP:0000278 | Retrognathia | 10.5 | 19.6 | HP:0009909 | Uplifted earlobe | 3.9 | 9.8 |
| HP:0011260 | Darwin notch of helix | 8.2 | 8.8 | HP:0004467 | Preauricular pit | 3.7 | 2.0 |
| HP:0000377 | Abnormality of the pinna | 8.2 | 26.5 | HP:0000957 | Cafe-au-lait spot | 3.3 | 2.0 |
| HP:0000322 | Short philtrum | 7.8 | 7.8 | HP:0008577 | Underfolded helix | 3.3 | 5.9 |
| HP:0000463 | Anteverted nares | 7.6 | 8.8 | HP:0002558 | Supernumerary nipple | 3.3 | 2.0 |
| HP:0009890 | High anterior hairline | 7.5 | 8.8 | HP:0000378 | Cupped ear | 2.8 | 2.0 |
| HP:0000337 | Broad forehead | 7.1 | 7.8 | HP:0000286 | Epicanthus | 2.4 | 2.9 |
| HP:0000582 | Upslanted palpebral fissure | 6.5 | 6.9 | HP:0000343 | Long philtrum | 2.4 | 6.9 |
| HP:0000316 | Hypertelorism | 5.5 | 11.8 | HP:0000219 | Thin upper lip vermilion | 2.2 | 6.9 |
| HP:0006610 | Wide intermammillary distance | 5.5 | 10.8 | HP:0000276 | Long face | 2.2 | 6.9 |
| HP:0000396 | Overfolded helix | 5.1 | 3.9 | HP:0011232 | Infra-orbital fold | 1.9 | 3.9 |
| HP:0000347 | Micrognathia | 4.3 | 2.0 | HP:0011262 | Crimped helix | 1.9 | 2.9 |
| HP:0002007 | Frontal bossing | 4.0 | 7.8 | HP:0001162 | Postaxial hand polydactyly | 1.8 | 1.0 |
| HP:0030084 | Clinodactyly | 1.8 | 7.8 | ||||
| HP:0011263 | Forward facing earlobe | 1.5 | 2.0 | ||||
| HP:0000331 | Short/small chin | 1.5 | 8.8 | ||||
| HP:0000400 | Large ears/Macrotia | 1.4 | 1.0 | ||||
| HP:0009748 | Large earlobe | 1.4 | 2.0 | ||||
| HP:0002562 | Low-set nipples | 1.2 | 1.0 | ||||
| HP:0000293 | Full cheeks | 1.1 | 2.0 | ||||
| HP:0100015 | Stahl ear | 1.1 | 1.0 | ||||
| HP:0000325 | Triangular face | 1.0 | 3.9 | ||||
| HP:0000369 | Low-set ears | 0.8 | 2.0 | ||||
| HP:0012812 | Fullness of paranasal tissue | 0.7 | 3.9 | ||||
| HP:0005590 | Spotty skin hypopigmentation | 0.6 | 1.0 | ||||
| HP:0000154 | Wide mouth | 0.6 | 1.0 | ||||
| HP:0001159 | Syndactyly | 0.6 | 3.9 | ||||
| HP:0400004 | Long ear | 0.4 | 2.0 | ||||
| HP:0000054 | Micropenis | 0.4 | 1.0 | ||||
| HP:0000470 | Short neck | 0.4 | 2.9 | ||||
| HP:0002002 | Deep philtrum | 0.4 | 2.0 | ||||
| HP:0005585 | Spotty hyperpigmentation | 0.4 | 4.9 | ||||
| HP:0000954 | Single transverse palmar crease | 0.3 | 1.0 | ||||
| HP:0000387 | Absent earlobe | 0.3 | 1.0 | ||||
| HP:0000506 | Telecanthus | 0.3 | 2.0 | ||||
| HP:0000411 | Protruding ear | 0.1 | 1.0 | ||||
| HP:0011233 | Antihelical shelf | – | 1.0 | ||||
| HP:0000893 | Bulging chest | – | 1.0 | ||||
| HP:0000494 | Downslanted palpebral fissure | – | 1.0 | ||||
| HP:0000520 | Exophthalmos | – | 1.0 | ||||
| HP:0000324 | Facial asymmetry | – | 1.0 | ||||
| HP:0001380 | Ligamentous laxity | – | 1.0 | ||||
| HP:0000485 | Megalocornea | – | 1.0 | ||||
| HP:0001059 | Neck pterygium | – | 1.0 | ||||
| HP:0030676 | Satyr ear | – | 3.9 | ||||
| HP:0006665 | Short chest (coat hanger sign of ribs) | – | 1.0 | ||||
| Flattened base of the nose | – | 1.0 | |||||
| Short eyes | – | 1.0 | |||||
| Tubercle in the breast | – | 1.0 | |||||
- a REF, prevalence (%) in the reference population (9); NICU, prevalence (%) in the NICU group.
About 30% (n = 31) of newborns in the NICU had no minor anomalies, while another 30% (n = 31) had one, 22.5% (n = 23) two, and 16.7% (n = 17) three or more minor anomalies (nine newborns with 3, four with 4, three with 5, and one with 8 minor anomalies). Dysmorphism (three or more minor anomalies) was not related to the sex (OR in boys 0.94; 95%CI 0.33–2.83; p = .9), maternal age (OR 0.99; 95%CI 0.90–1.08; p = .8), paternal age (OR 1.00; 95%CI 0.93–1.07; p = .9), exposure to alcohol (OR 0.36; 95%CI 0.02–2.06; p = .3) or maternal infections during pregnancy (OR 1.12; 0.33–3.40; p = .9).
3.3 Major anomalies
A total of 19 major anomalies were observed in 16 newborns (15.7%), including clubfoot (n = 4), spina bifida (n = 1), duodenal stenosis (n = 1), gastroschisis (n = 2), agenesis of lacrimal puncti (n = 1), congenital heart defect (n = 6), anorectal malformation (n = 1), anencephaly (n = 1), myelomeningocele (n = 1), hydrocephaly (n = 1). Three children presented with two major anomalies, and two children with three major anomalies. The risk of having a major anomaly increased significantly with the number of minor anomalies in this sample, even though the number of children is relatively small (Figure 1; OR: 1.43; 95%CI: 1.02–2.05; p = .04). Adjustment for the birthweight z-score, gestational age and age of the mother had little impact on this result, and neither factor was significantly associated with the presence of a major malformation.
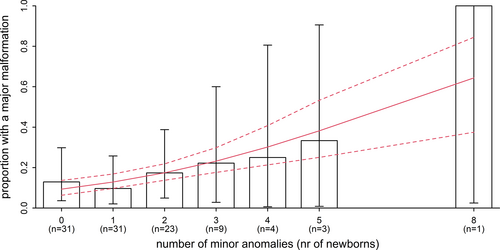
The number of minor anomalies was not significantly associated with any of the other common reasons for admittance: infection (OR 0.94; 95%CI: 0.68–1.35), asphyxia (OR 0.78; 95%CI: 0.51–1.11), haemolytic disease (OR 1.00; 95%CI: 0.68–1.40), intraventricular hemorrhage (OR 0.95; 95%CI: 0.58–1.39) and other (OR 1.03; 95%CI: 0.76–1.37).
Of the 16 newborns with major anomalies, 11 had an isolated major anomaly. Of the five newborns with a major anomaly with dysmorphism, none represented a recognizable syndrome. Of the 12 newborns with dysmorphism, one child had the characteristics of Down syndrome.
3.4 Survival
Of the 102 newborns recruited, 30 (29.4%) died during the hospitalization (n = 29) or follow-up after discharge (n = 1), and six were lost to follow-up. Among these were 17 males (28.6% of males), 11 females (27.5% of females) and the conjoined pair of twins with ambiguous external genitalia, and 10 were premature (34.5% of premature) and 20 term (27.4% of term) newborns (OR in premature 1.39; 95%CI 0.54–3.48). The reason for admission in the 30 children who died was a major anomaly (n = 13, 43.3%), neonatal infection (n = 26, 86.6%), intraventricular hemorrhage (n = 8, 26.6%) or asphyxia (n = 5, 16.6%), hemolytic disease (n = 5, 16.6%) or other (n = 9, 30%). Of these, 26 (86.7%) had more than one reason for admission.
Death occurred during the first 7 days after birth in 16 newborns (53.3%) and another six (20%) died during the second week (Figure 2). Of the newborns discharged (n = 72), follow-up data at 1 year after discharge were available on 66. Of these, another one died before the age of 1 year.
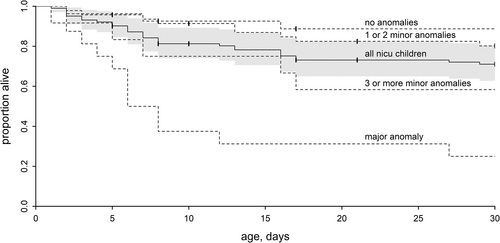
In newborns without dysmorphism (i.e., <3 minor anomalies) and no major anomaly (n = 74), survival at the end of follow-up was 80.8%. For those with three or more minor anomalies but no major anomalies (n = 12) survival was 58.3%. For newborns with at least one major anomaly (n = 16), survival was 25% at 1 months and 18.7% at 1 year. Those who survived had a clubfoot or duodenum stenosis as major anomaly.
The presence of a major anomaly was associated with a hazard ratio of death of 13.2 (95%CI 3.7–46.7; p < .001; Figure 2 and Table 2). The presence of three or more minor anomalies was associated with a 4.5-fold increased risk of death (95%CI 1.1–18.6; p = .04). The hazard ratios are only slightly affected when adjusting for birth weight and gestational age, and the level of significance remains.
| Survival (95%CI) | Hazard ratio (95%CI) | p | |
|---|---|---|---|
| No anomalies | 88.7% (77.5–100%) | Reference | – |
| 1–2 minor anomalies | 80.2% (69.4–92.7%) | 1.8 (0.5–6.5) | .4 |
| 3 or more anomalies | 58.3% (36.2–94.1%) | 4.5 (1.1–18.6) | .04 |
| Malformation | 18.8% (6.8–52.0%) | 13.2 (3.7–46.7) | <.001 |
| All | 70.0% (61.5–79.6%) | – | – |
4 DISCUSSION
Limited knowledge exists on the outcome of newborns with major anomalies and/or multiple minor anomalies in low- and middle-income countries. The obvious reason is that emphasis is still on infectious diseases or asphyxia as major causes of perinatal morbidity and mortality. According to the Health Newborn Network, worldwide, 80% of newborn mortality is related to prematurity, birth-related complications and infections, and 11% due to congenital anomalies. In the DR Congo, congenital anomalies are estimated to explain 7% of newborn mortality (https://www.healthynewbornnetwork.org/). However, advances in perinatal care are expected to result in a proportionately larger impact of congenital anomalies. The decline in neonatal mortality seen worldwide due to of infections and neonatal intrapartum-related events is not paralleled by a decline in congenital anomaly–related mortality (Liu et al., 2016). We therefore prospectively studied a cohort of newborns admitted to the NICU of a reference hospital in Kinshasa, capital of the DR Congo in Central Africa. This cohort was specifically studied since it was more likely to be enriched in major anomalies and thus being more informative. Indeed, in this cohort the incidence of major anomalies was 15.6%, which is higher compared to the incidence of 2.2% in an unselected population of newborns in the same city (Mubungu et al., 2020). This figure may underestimate the true prevalence of major anomalies, since access to imaging of the internal organs is highly restricted in the DR Congo and only performed when there are clinical indications for specific malformations. Moreover, as in many low-income countries, no social security exists, and patients have to pay themselves for each diagnostic intervention.
Less than 1 in five newborns admitted to the NICU with a major anomaly did survive beyond 1 year. The probability of survival after 1 year in newborns with a major anomaly is approximately on fifth of that in newborns without major anomaly and no dysmorphism. This confirms previous observations that newborns with congenital anomalies constitute a highly vulnerable population, with a poor prognosis, in this setting. The present cohort of newborns is not representative of all newborns with a major anomaly born in Kinshasa. Newborns admitted to the NICU often present additional problems including as asphyxia or infections (observed in 23 and 82 cases, respectively). In contrast, the majority of newborns with congenital anomalies transferred to the University Hospitals are admitted to the pediatric surgery unit when they do not present such additional complications. Further research in that group can therefore complement the present study. Also, newborns presenting severe or multiple congenital anomalies are underrepresented in this study, because often they are already deceased before transfer to the NICU.
This observation corroborates the poor outcome of congenital anomalies in Central Africa, especially when associated with other risk factors. Studies from other African countries reported a variable outcome for congenital anomalies, with mortality ranging from 10% in Eritrea and Nigeria (Ajao & Adeoye, 2019; Andegiorgish et al., 2020; Shah, Zemichael, & Meng, 2012) to around 50% in Cameroon (Mah Mungyeh et al., 2014). However, differences in the characteristics of admitted neonates makes a comparison difficult.
We also noted that the presence of three or more minor anomalies is associated with a significant four-fold increased risk of death during the first month, compared to newborns without minor or major anomalies. Minor anomalies have no functional consequences, but, since they are errors of morphogenesis, they indicate a disturbance of embryonic or fetal development. It is likely that some of the newborns with multiple minor anomalies in this study have an underlying syndrome, which was not yet clinically recognized. In the present cohort, the incidence of multiple (three or more) minor anomalies was 16.7%, which is significantly higher (p < .001) than the 4.3% observed in a reference population by the same investigator (G.M.; Mubungu et al., 2020). This indicates that children with dysmorphism equally represent a vulnerable population, with an increased risk of being admitted to the NICU. Previous studies have shown that the presence of minor anomalies is associated with a higher risk of a major anomaly (Hennekam, 2011). In the present study, we equally observed such a relationship, with each additional minor anomaly representing a 40% increased chance of having a major anomaly.
In this cohort of 102 newborns, we observed 13 minor anomalies that were not present in a reference cohort of 722 newborns. This is not unexpected, given the large number of possible minor anomalies. On the other hand, certain minor anomalies might be more prevalent in certain syndromes, and thus enriched in a NICU population. Of these 13 minor anomalies not observed in the general population, seven were observed in newborns with three or more minor anomalies, of whom two also had a major anomaly.
The impact of congenital anomalies may also be related to the etiology of the disorder. In this cohort, only one of the newborns with dysmorphism or with multiple major anomalies presented a recognizable disorder, that is, Down syndrome. For the other children, reaching an etiological diagnosis was hampered by the limited access to technical investigations such as ultrasound or radiological imaging, biochemical analyses, and lack of genetic testing.
Long-term follow-up of patients is challenging in the DR Congo. In our previous study of newborns examined in maternities, most newborns with anomalies were lost to follow-up. In contrast, in the present study, only 6/72 newborns were lost to follow-up at the age of 1 year (8.3%). This is likely due to the highly selected group of patients, with a high interest by the parents in medical follow-up.
A limitation of this study is the small sample size. Over a period of 2 years, we recruited only 102 newborns in the NICU. This limited number is due to the low number of children born in the University Hospitals, of which only few need admissions to the NICU. Moreover, given the distant location of the University Hospital from the City Center, only few external referrals occur.
In conclusion, major anomalies and multiple minor anomalies are important predictors of outcome in newborns admitted to a NICU in a low-income country. This underscores the need for training clinicians to recognize and interpret minor and major anomalies.
ACKNOWLEDGMENTS
The authors are thankful to children and their parents for their participation to this study, and to the staffs of the Neonatology Intensive Care Unit of the University Hospitals for their great support. The authors are also thankful to Dr Fwanani Patrick, Dr Mbala Bedie for their assistance during data collection. Marc Vervenne Fund Doctoral Scholarship, KU Leuven. Chair for Human Genetics in DR Congo, KU Leuven.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Gerrye Mubungu contributed to the protocol design, data collection, data interpretation, writing, and revision of the report. Nono Mvuama contributed to data analysis and revision of the report. Dahlie Tshika and Prince Makay contributed to the data collection. Tady Bruno and Thérèse Biselele contributed to the revision of the report. Mathieu Roelants contributed to data analysis, writing and revision of the report. Koenraad Devriendt, Prosper Lukusa-Tshilobo, and Aimé Lumaka contributed to the protocol design, data interpretation, writing, and revision of the report. All authors have read and approved the final version.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




