Mucopolysaccharidosis type I newborn screening: Importance of second tier testing for ethnically diverse populations
Abstract
Mucopolysaccharidosis type I (MPS I)/Hurler syndrome newborn screening was added to the recommended uniform screening panel (RUSP) in 2016. As states have added screening for MPS I, programs have reported increased rates of false positives. Reasons for false positive screens include carrier status, true false positive, late-onset/attenuated forms, and in about half of cases, pseudodeficiency alleles. These alleles have DNA variants that can cause falsely decreased enzyme activity on biochemical enzyme studies and have increased frequency in individuals of African American and African descent. We describe the District of Columbia (DC) experience with MPS I screening from December 2017 to February 2019. In the context of a review of the literature on newborn screening and family experiences and this DC-based experience, we offer potential solutions to address preliminary concerns regarding this screening. The impact of overrepresentation of screen positives in a minority group and unintentional creation of health disparities and community wariness regarding medical genetics evaluations must be considered to improve the newborn screen programs nationally and internationally.
1 INTRODUCTION
Newborn screening is an effective tool to identify newborns at risk of treatable disorders; prevent complications; and, in some cases, deliver life-saving medical management in a timely manner. Identifying true positives in a population can be challenging due to a broad range of biochemical cut-offs, barriers to follow-up testing, limited access to genetic sequencing, and lack of available genetic providers with knowledge on interpretation of results. Differences in metabolic screening markers may vary based on the infants' ethnic background resulting in false-positive reporting (Peng et al., 2020; Wasserstein et al., 2019).
The mucopolysaccharidoses (MPSs) are a group of progressive genetic disorders of glycosaminoglycan (GAG) metabolism caused by deficiency of enzymes responsible for lysosomal GAG degradation. The accumulation of partially degraded GAGs and the resulting disturbance of cellular homeostasis leads to progressive cellular and tissue damage ultimately resulting in multi-organ system involvement (Clarke, 1993-2020; Heath Resources and Services Administration, 2019).
MPS I was traditionally classified into the types Hurler, Hurler–Scheie, or Scheie; however, it is now known that MPS I exists on a spectrum ranging from severe to mild, labeled severe and attenuated. Severe MPS I has a chronic and progressive disease course involving multiple organs and causes joint disease, cardiorespiratory compromise, and death by 18 months if not treated. Attenuated MPS I exhibits similar symptoms; however, the rate of progression and severity of complications is delayed and patients rarely show neurological involvement beyond learning disabilities (Clarke et al., 1993-2020). MPS I is inherited in an autosomal recessive manner and has a recorded incidence of 0.54–1.84 cases per 100,000 newborns (Kemper, 2015).
Mucopolysaccharidosis type I (MPS I) newborn screening was added to the recommended uniform screening panel (RUSP) in 2016 (Heath Resources and Services Administration, 2019). The RUSP is a list of disorders that the Secretary of the Department of Health and Human Services (HHS) recommends for states to screen as part of their state universal newborn screening (NBS) programs (Millington et al., 2018; Watson, 2006). Disorders are chosen based on evidence that supports the potential net benefit of screening, the ability of states to screen for the disorder, and the availability of effective treatments (Heath Resources and Services Administration, 2019). Currently, two modalities are used for screening for lysosomal storage disorders, Liquid Chromatography with tandem mass spectrometry (LC-MS/MS) or microfluidics. Each state determines the modality appropriate for their state's needs and each has its benefits (reviewed in Millington et al., 2018). Included in the proposed algorithm for MPS I newborn screening is molecular testing which is confirmatory and may or may not be adopted into the newborn screening framework, based on cost and availability. Identification of attenuated forms on initial positive screen can be difficult. The presence of a molecular result consistent with a known pathogenic change associated with severe or attenuated disease would lead to counseling regarding disease progression and treatment. A genetic result of a pseudodeficiency change would lead to no medical intervention and family support for the anxiety caused by the abnormal newborn screen process (reviewed in Tu, He, Chen, Shi, & Li, 2012; O'Connor et al., 2018).
Early presymptomatic detection of MPS I via newborn screening may result in improved neurocognitive outcomes through earlier enzymatic replacement therapy (ERT) and hematopoietic stem cell transplant (HSCT), even though improvement in mortality has not been established (Kemper, 2015). HSCT is the standard of care for infants diagnosed with severe MPS I and is recommended before the age of 2. Even with limited evidence, MPS I is perceived by health care providers as a favorable condition to screen due to improved efficacy and availability of therapeutic options (Lisi & McCandless, 2016). This makes early identification of MPS I via newborn screening fit RUSP criteria of an available treatment which requires presymptomatic initiation.
When considering false positive screens, analysis of prospective lysosomal storage disease (LSD) newborn screening data from Illinois and Missouri both revealed that pseudodeficiency alleles contributed to the high false positive rate in screening for MPS I (Millington, 2018). As per previous experience in other states that screened for MPS I, out of 40 positive newborn screens, only 1 patient had a true diagnosis (Burton et al., 2017). Reasons for false positive screens included carrier status, enzyme level initially abnormal but on repeat normal, and pseudodeficiency alleles in about half of cases. Thus, pseudodeficiency alleles account for approximately 50% of all newborn screening referrals for MPS I (NewSteps, 2018). Pseudodeficiency alleles are DNA variants that can cause decreased enzyme activity on in vitro biochemical enzyme studies, which do not cause disease nor lead to clinical manifestations. In many cases, enzyme studies, such as the ones used in newborn screening, cannot differentiate between truly affected infant or those harboring pseudodeficiency variants. These individuals require additional testing to confirm the diagnosis. Although described as rare in the literature, pseudodeficiency alleles in MPS I are quite common in African Americans and those of African descent (Kemper et al., 2015); albeit an exact prevalence has not been published to date. This is especially relevant to our population, as 46.4% of individuals identified themselves as black/African American alone in the District of Columbia (DC) (from the United States Census Bureau published online July 2019).
In the case of MPS I newborn screening, when enzyme activity of α-L-iduronidase (IDUA) is inconclusive and genetic sequencing is not available, a second approach is to use confirmatory testing including measurement of blood and/or urine GAGs. A recent study published by (Stapleton et al., 2020) suggests that general screening of newborns by both blood GAGs and enzyme assays may be a sensitive two-tiered strategy for the diagnosis of MPS patients, this is yet to be published by other groups. Multiple groups have shown that blood GAG levels may be sensitive markers for various MPS types (de Ruijter et al., 2012; Shimada et al., 2014; Tomatsu et al., 2010; Tomatsu et al., 2013). de Ruijter et al. (2012) demonstrated elevation of blood GAGs in newborn blood spots from the Dutch NBS program in 11 MPS I patients including attenuated patients, compared to unaffected newborns. Some have suggested using GAG levels as first tier screening followed by enzyme levels as a second tier screen. Given the heterogenicity of the condition, it is possible to miss an attenuated forms of MPS I if only blood and urine GAGs are completed without enzymatic or molecular confirmation.
We describe the DC experience from December 2017 to February 2019 for newborn screening for MPS I and offer potential solutions to address preliminary concerns regarding overrepresentation of screen positives in a minority group and unintentional creation of health disparities and community wariness regarding medical genetics evaluations (Ross, 2008).
2 MATERIALS AND METHODS AND RESULTS
2.1 Methods
The DC joined the group of 16 States screening for LSDs including MPS I. 2018 data from DC newborn screening program reported a total of 15,235 samples from approximately 14,665 newborns that were submitted from 27 District submitters including the region's five major birth hospitals, to PerkinElmer Laboratory. The Washington DC program currently utilizes PerkinElmer for lysosomal storage disease newborn screening (NeoLSD) that uses Flow Injection Analysis Tandem Mass Spectrometry (FIA-MS/MS) to determine enzymatic activity.
At the time of writing, the proposed algorithm for MPS I newborn screening adopted by various newborn screening programs including our group involves a combination of the following: (a) Low IDUA enzyme is detected on dried blood spot; (b) a follow-up blood sample for leucocyte enzyme analysis is performed; (c) follow-up urine GAG and/or blood GAG analysis; and (d) some individuals have confirmatory molecular testing for IDUA variants, based on access to testing. The last step of molecular testing is confirmatory and may or may not be adopted into the newborn screening framework, based on cost and availability. Recently, IDUA molecular testing was made as an automatic second tier test using dried blood spots (DBS) collected during the newborn screen for all infants in Washington, DC area based on our initial experience described here.
Confirmatory blood GAGs, urine GAGs, and IDUA levels were analyzed by the Mayo Clinical Laboratories. Their clinical website describes their unpublished methods as using LC-MS/MS (https://www.mayocliniclabs.com/).
Data collected from December 2017 to February 2019 by Children's National Hospital, a referral center for all MPS I positive newborn screens in Washington, DC was analyzed retrospectively for all patients, with and without molecular testing and separated based on self-reported ethnicity which was documented in the patient chart. This study was performed under IRB approval (Children's National Hospital IRB). Data sharing is protected by the IRB and is available through collaboration.
2.2 Results
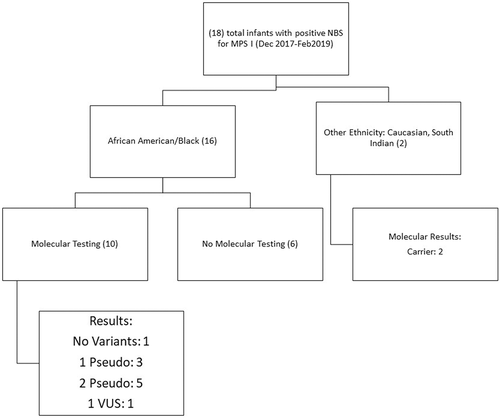
The findings of this chart review were as follows: 16/18 patients identified by the program were of African American/Afro-Caribbean/African descent (one patient identified as half “black” half “Hispanic”). Each infant was followed up with a blood sample for leucocyte enzyme analysis (DBS-IDUA), urine GAGs, and blood GAGs. The results were as follows for 16 infants of African American/black/Afro-Caribbean/African descent: blood GAGs were normal for all infants. Three infants showed elevated total urine GAGs; however, individual GAG species were normal and one infant showed normal total excretion of urine GAGs and mildly elevated excretion of dermatan sulfate. Eleven infants had low confirmatory IDUA enzyme activity (DBS-IDUA) and five had normal enzyme activity. Genotypes for the infants were as follows: three heterozygous pseudodeficiency (one also had a VOUS), three compound heterozygous pseudodeficiency alleles, two homozygous pseudodeficiency, one heterozygous VOUS, and one negative result (Figure 1). Five infants did not have molecular testing due to insurance denial of coverage and one infant was lost to follow-up. In this limited group, eight patients of African American descent who screened positive and had molecular testing had pseudodeficiency alleles (80%), and of the pseudodeficiency alleles found, c.235G>A (p.Ala79Thr), c.246C>G (p.His82Gln), c.667G>A (p.Asp223Asn), and c.965T>A (p.Val322Glu) were noted to occur more frequently in the population of African descent, 30, 5, 20, and 5%, respectively (compared to no pseudodeficiency identified in the non-African descent population). No pathogenic alleles were found in the cohort of African descent (Table 1). The two additional babies identified in the screening process were found to be carriers of MPS I on genetic sequencing.
| Allele | Classification | Times detected (patient n = 12, allele n = 24) | Percentage of alleles in screen positive population | Percentage of alleles in screen positive population of African descent |
|---|---|---|---|---|
| c.235G>A (p.Ala79Thr) | Pseudodeficiency | 6 | 25 | 30 |
| c.246C>G (p.His82Gln) | Pseudodeficiency | 1 | 4.2 | 5 |
| c.667G>A (p.Asp223Asn) | Pseudodeficiency | 4 | 16.6 | 20 |
| c.965T>A (p.Val322Glu) | Pseudodeficiency | 1 | 4.2 | 5 |
| c.979G>C (p.Ala327Pro) | Pathogenic | 1 | 4.2 | 0 |
| c.1205G>A (p.Trp402X) | Pathogenic | 1 | 4.2 | 0 |
| c.1577T>C (p.Gly582Lys) | VUS | 1 | 4.2 | 5 |
| c.1744G>A (p.Glu582Lys) | VUS | 1 | 4.2 | 5 |
- Note: Note the pseudodeficiency alleles account for 90% of the variants found in patients of African descent; 80% of patients of African descent had at least one pseudodeficiency allele. The non-African patients were both carriers.
- Abbreviation: VUS, variant of unknown significance.
2.3 Limitations
Out of 16 African American infants who screened positive, 6 did not have molecular testing, including 3 who had abnormal confirmatory enzyme level. These infants were assumed to be carriers of pseudodeficiency alleles based on negative blood and urine GAGs. Recent literature is reassuring that this conclusion was appropriate for patient care (Stapleton et al., 2020).
3 DISCUSSION
3.1 Pseudodeficiency alleles
The pseudodeficiency allele frequency for Washington, DC, data presented in Table 1 is higher than previously published populations (summarized in Table 2). States that documented ethnicity (Illinois, NC) showed a strikingly high pseudodeficiency rate in the African and/or African American cohorts (26/28 and 13/13, respectively). The New York data from an “ethnically diverse” population, pseudodeficiency was reported for a 8/13 false positive screens (Wasserstein et al., 2019). This shows that the populations of African descent are at a higher risk for abnormal newborn screen secondary to pseudodeficiency than other populations. In our study all of pseudodeficiency alleles were in individuals of African American/Afro Caribbean/African descent, consistent with ethnicity percentages in the DC. Nearly 90% of our screen positive patients identify as African descent, a disparity considering 46% of the city identifies as black. The much higher rate of pseudodeficiency in the Washington, DC cohort is most likely due to the higher proportion of those of African descent. In comparison, the North Carolina Census data shows a 22.2% of individuals that self-identify as black/African American. Thus, the correlation between self-identified ethnicity and risk of false positive newborn screen secondary to pseudodeficiency is striking.
| Illinois (Nov 2014–Aug 2016) (Burton et al., 2017) | Missouri (Jan 2013–Dec 2014) (Kemper et al., 2015) | North Carolina (Aug 2016–Mar 2017) (Taylor et al., 2019) | New York (May 2015–) (Wasserstein et al., 2019) | Washington, DC (Dec 2017–Feb 2019) | |
|---|---|---|---|---|---|
| Screen positive samples | 151 of 219,713 | 70 of 174,636 | 54 of 62,734 | 13 of 35,816 | 18 of 14,665 |
| Patients with MPS I disease (2 pathogenic variants) | 1 | 1 | 1 | 0 | 0 |
| Carriers (1 variant) | 5 | 3 | 2 | 4 | 2 |
| Patients with pseudodeficiency allele(s) | 28 | 25 | 13 | 8 | 8 |
| Patients with a false positive screen | 87 | 30 | Not reported | Not reported | 2 |
| Number of AA with pseudodeficiency alleles | 26 | Not reported | 13 | Not reported | 8 |
The importance of ethnicity and false positive rates are increasingly a concern in the metabolism community. Based on previous reports of increased stress and guilt associated with false positive results on newborn screen (reviewed in O'Connor et al., 2018) and the history of mistrust of genetic testing within the most affected populations (Ross, 2008), the importance of screens including secondary mechanisms to reduce false positive rates is essential to build trust. Furthermore, the field is accumulating evidence of many types of markers and the ethnic risk for false positive rates (described and reviewed in Peng et al., 2020). Thus, this work is another piece of the complex puzzle to create a newborn screen that ensures the highest sensitivity and specificity possible for each newborn.
3.2 Learning from past and present experiences: Newborn screening in minority groups
Newborn screen was designed to offer all newborns the same access to screening—regardless of ethnicity, socioeconomic status, or location in a state. In current times, to have newborn screen results that disproportionately affect one population is disheartening and must be remedied. The above findings that the majority of our screened positive infants were of African descent raised a few considerations: (a) Are we inadvertently creating health disparities in a single ethnic group? (b) Are state newborn screening programs equipped to deal with large numbers of positive screens from a single ethnic group?, and (c) Should all states require second tier testing (either GAGs or sequencing) to reduce false positive rates? In the 3 years since beginning this work, the interventions, publications, and discussions at national meetings have shown that the field is ready to take action in order to decrease these healthcare disparities, and therefore, states have added second tier testing through either molecular sequencing or biochemical analysis (GAG measurement using the NBS DBS). However, the best approach to change is complicated. To understand this, we must consider health disparities and identify a model that has worked for other populations with a high risk of complex newborn screen results. Health disparities are differences in health or its key determinants that may adversely affect marginalized or excluded groups. As NBS programs evolve, there has been an increased call to ensure that they continue to reduce the persistent health disparities among historically underserved populations (Brosco, Grosse, & Ross, 2015). Newborn screening is a system that begins with testing but succeeds with adequate follow-up and counseling. When all NBS conditions on the RUSP are combined, including hearing loss and critical congenital heart disease, approximately 5 in 1,000 newborns have a condition detectable by screening that can be addressed (Brosco, Grosse, & Ross, 2015). Therefore, the program is one of the greatest public health triumphs. At the same time, adequate follow-up relies on confirmatory testing, and when costs are associated and not all families have equal insurance coverage, some patients may disproportionately bear the price and financial burden or lose access to positive and even life-saving interventions. If the system of newborn screening is dependent on a patient's social situation in which the infant and family receives lesser care, this creates a discord between the system and population which it was designed to serve. In this case some infants/families received genetic testing while others did not due to insurance coverage. When this preliminary data was presented to the DC, the newborn screen program was updated to include second tier testing. By quickly implementing the second tier molecular testing, the District showed its commitment to supporting equitable screening for all neonates.
The goal of the metabolic group in DC is to elevate the respect for metabolic screening to that of the sickle cell screen in the African American population. Newborn screening for sickle cell anemia can reveal both carriers and those affected by the condition. This is not unique to sickle cell anemia screening only; however, the identification of carriers has proven to be beneficial. Its inclusion on newborn screening has helped to decrease health disparities by providing timely diagnosis and interventions. Sickle cell disease occurs in about 1:500 African Americans, and sickle cell trait affects about 1:12 African Americans and 1:20 Dominicans. The higher prevalence of both sickle cell disease and sickle cell carriers in the African American community often also results in high screen positive rates, since both the carrier and disease state screen positive. While the carrier state is not immediately actionable in the newborn period, useful information to the family includes potential consideration of partner testing for future pregnancies, and knowledge for the child later on in life (Bender, 1993). Group sessions, which included African American adults with varied educational backgrounds, examined views regarding sickle cell screening. During this session, sentiments of distrust of the medical profession, due to past abuses of African Americans, a concern previously raised in the medical literature, also resurfaced (Brosco, Grosse, & Ross, 2015). Participants of a more recent study, which focused on awareness of sickle cell anemia in the African American and Dominican communities, expressed that people would prefer face-to-face counseling from doctors or provision of internet material rather than telephone calls from providers or counseling from health educators (Siddiqui et al., 2012). However, when provided with more information, the group viewed newborn screening as overall beneficial (Long, Thomas, Grubs, Gettig, & Krishnamurti, 2011). Thus, using the model of the sickle cell screening program and how they have approached health literacy and their work toward health equity is essential. This screening program incorporated intervention and education and offers an example of the way that the metabolic field may better support this same population when facing the highest risk for false positive results in MPS I testing.
3.3 Newborn screen: The family screen
Newborn screening results can have far reaching effects, which not only pertain to the infant evaluated, but also to the entire family. Potential impacts on the family include feelings of guilt, confusion, and regret over the choice of a particular partner and sometimes stigmatization of the mother (Marsh, Kamuya, & Molyneux, 2011). In addition, direct family members, including siblings and parents, may find out that they are either carriers of a condition or even affected. In this aspect, newborn screening is in reality a “family screen,” with many complex factors that go into initial evaluation and follow-up visits. Pruniski, Lisi, & Ali (2018) evaluated the impact of newborn screening of Pompe disease on families. Even though this publication was limited by the reported size and population that was surveyed, parental reactions to newborn screening reported increase in fear/anxiety and living with uncertainty in addition to concerns about social and healthcare related topics. A similar study for MPS I within our population is needed.
To mitigate the potential adverse effects of false positive and screen positive results, particularly in ethnically diverse populations, future research should focus on development of more culturally sensitive and specific materials to improve patient and community engagement and also continue to track the effect of false positives on this community. Hall and Opolade wrote, “Discovering a condition in the newborn period is not sufficient to eliminate disparities in outcomes.” They go on to say that “special attention to historically underserved populations, including targeted interventions to improve short-term follow-up, may be needed to ensure that the benefits of early identification are universally obtained” (Hall & Olopade, 2006).
By leveraging the most recent research showing this health disparity and the research showing approaches to minimize the false positive rates (i.e., sequencing or GAG evaluation), we now have the tools to improve the newborn screening system to what the goal is—a screen that equalizes all babies for one moment in their lives. This can only occur if all infants have access to the same prenatal and postnatal care. Our preliminary data revealed that only some, but not all infants, who initially screened positive, had follow-up molecular testing completed. The main barrier was denial of molecular testing by federal/state insurance programs because the test was not covered in five patients. One family/patient was not insured/self-pay, refused testing, and was lost to follow up. Based on our review of the literature and the DC experience, we advocate that MPS I secondary testing should be performed in conjunction with DBS-IDUA activity levels on all infants as part of the initial newborn screen, regardless of race, ethnicity, or ability to pay. This view has been mirrored by the experience in the North Carolina program, the Georgia program, and the District of Columbia program when this preliminary data was shared. In each case the programs implemented molecular testing to offer families a definitive modality to confirm problematic pseudodeficiency findings (Hall et al., 2020; Taylor et al., 2019).
By carefully tracking neonates as they progress through the newborn screening systems, acknowledging science's limitations, listening to family's fears and frustrations, and finding new ways to improve patient care every day, newborn screen will continue to work toward more equitable outcomes for all babies, regardless of their state, ethnicity, income, or access to resources.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Kerri Bosfield: Performed data analysis, first article draft, and patient care. Debra S. Regier: Performed data analysis, final article editing, and patient care. Sarah Viall: Performed data analysis and patient care. Rebecca Hicks: Performed data analysis and drafted sections of the article. Natasha Shur: Performed data analysis, final article editing, and patient care. Christina L. Grant: Performed data analysis, patient care, and final article editing.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is protected by the IRB and is available through collaboration.




