Ultra-rapid emergency genomic diagnosis of Donahue syndrome in a preterm infant within 17 hours
Funding information: Deutsche Forschungsgemeinschaft, Grant/Award Number: CI 218/1-1
Abstract
Genetic diseases are a major cause of neonatal morbidity and mortality. The clinical differential diagnosis in severely ill neonates, especially in premature infants, is challenging. Next generation sequencing (NGS) diagnostics is a valuable tool, but the turnaround time is often too long to provide a diagnosis in the time needed for clinical guidance in newborn intensive care units (NICU). To minimize turnaround time, we developed an ultra-rapid whole genome sequencing pipeline and tested it in clinical practice. Our pilot case, was a preterm infant presenting with several crises of dehydration, hypoglycaemia and hyponatremia together with nephrocalcinosis and hypertrophic cardiomyopathy. Whole genome sequencing was performed using a paired-end 2x75bp protocol. Sequencing data were exported after 50 sequencing cycles for a first analysis. After run completion, the rapid-sequencing protocol, a second analysis of the 2 x 75 paired-end run was performed. Both analyses comprised read-mapping and SNP−/indel calling on an on-site Edico Genome DRAGEN server, followed by functional annotation and pathogenicity prediction using in-house scripts. After the first analysis within 17 h, the emergency ultra-rapid protocol identified two novel compound heterozygous variants in the insulin receptor gene (INSR), pathogenic variants in which cause Donohue Syndrome. The genetic diagnosis could be confirmed by detection of hyperinsulinism and patient care adjusted. Nonetheless, we decided to pursue RNA studies, proving the functional effect of the novel splice variant and reduced expression levels of INSR in patients skin fibroblasts.
1 INTRODUCTION
Genetic diseases are a major contributor to neonatal morbidity and mortality (Malam et al., 2017; Weiner, Sharma, Lantos, & Kilbride, 2011). The clinical differential diagnosis in severely ill neonates, particularly in premature infants, is extremely challenging (Pettinger et al., 2019) because diagnostic hallmarks of phenotypes are often not yet completely developed or could be masked by common illnesses in preterm neonates (Broekaert et al., 2018; Elliott et al., 2019; Kuehne et al., 2019). Even if the patients present with a distinct phenotype, genetic heterogeneity is frequent and makes a candidate gene approach difficult. Routine metabolic and conventional genetic diagnostics, such as karyotyping, Array-based Comparative Genomic Hybridization (Array-CGH) or single gene sequencing, may take several weeks or months and a significant amount of cases remain unsolved (Malam et al., 2017). Consequently, many patients with genetic disorders are discharged or die prior to diagnosis (Elliott et al., 2019).
Next generation sequencing (NGS) studies have led to a breakthrough in the identification of a great number of disease genes and become a part in clinical routine diagnostics in rare disease and/or disease entities with underlying genetic heterogeneity (Bamborschke et al., 2018; Boycott et al., 2017; Fakhro et al., 2019; Pergande et al., 2020). Noteworthy, several pilot studies have been performed over the last 8 years regarding the implementation of NGS in pediatric and newborn intensive care units (PICU/NICU), because in this setting an early genomic diagnosis can lead clinicians in management decisions and alter clinical outcome (Kingsmore et al., 2019; Malam et al., 2017; Meng et al., 2017; Stark et al., 2016). By improving sequencing techniques, computational data processing and variant analysis, the mean turnaround time has been shortened to 5–12 days, though multiple patients have been diagnosed significantly more rapidly than these mean timeframes, especially if sample transportation time was minimized by conducting sequencing on-site (Brunelli et al., 2019; Clark et al., 2019; Elliott et al., 2019; Farnaes et al., 2018; Kingsmore et al., 2019; Petrikin et al., 2018; Sanford, Wong, Ellsworth, Ingulli, & Kingsmore, 2020; Saunders et al., 2012; Stark et al., 2016; Stark et al., 2018). Despite all successes and multiple cases that have been solved more quickly, results in approximately 1 to 2 weeks remains too long in many cases to have an immediate impact on clinical care in the short critical time frame of newborn intensive care units (NICU). Here we present an ultra-rapid whole genome sequencing pipeline, which we tested successfully in a severe ill preterm infant and could reach the diagnosis within only 17 h.
2 MATERIAL AND METHODS
After informed consent, peripheral venous blood was drawn from the index patient and both parents, immediately transferred to the lab, and processed according to our ultra-rapid whole-genome sequencing (WGS) pipeline (Figure 1). Genomic DNA was extracted via QIAamp DNA Blood Mini Kit (Quiagen, Venlo, Neatherlands) following the manufacturers' protocol. Library preparation for the index patient was performed with the Nextrera DNA Flex Kit (Illumina, San Diego, California) followed by whole genome sequencing on three lanes of the Illumina HiSeq 4,000 sequencing instrument using a 2x75bp protocol. For early preliminary results, sequencing data were exported after 50 sequencing cycles for a first analysis. After run completion, a second analysis of the 2x75 paired-end run was performed. Alignment and variant calling were done on an onsite Edico Genome (now part of Illumina) DRAGEN system and the Cologne Center for Genomics' VARBANK pipeline v.2.12 (https://varbank.ccg.uni-koeln.de/) was used for annotation and pathogenicity prediction, as described (Pergande et al., 2020; Wang et al., 2017). The analysis was restricted to the target regions of the Agilent SureSelect Human All Exon V6 exome sequencing kit (Agilent, Santa Clara, California). Common variants with an allele frequency of >0.1% in 1000 Genomes (www.internationalgenome.org) and deep intronic variants were excluded, as well as variants annotated frequently in our in-house database as indication of a pipeline artifact. The remaining variants were then manually curated by gene function, known disease association and severity of the predicted effect on the protein product using the ensemble variant effect predictor (www.ensembl.org). In the first step we perform an analysis and pathogenicity prediction of each variant. In the second step these variants are evaluated in combination with the reported phenotype. By this approach, compared to clinical panel testing new phenotype associations or expansions of the phenotype of known-disease causing genes could be ascertained and variants causing atypical phenotypes are more unlikely to be missed. In known disease genes, the classification of the American College of Medical Genetics and Genomics (ACMG) (Richards et al., 2015) and the recently introduced two-dimensional system for variant classification of the European Society of Human Genetics (ESHG) (https://www.eshg.org/index.php?id=949) were applied. For the main resulting variants primer were designed and ordered overnight. Confirmation of the variants was performed the following day by Sanger Sequencing of the index patient and its parents.
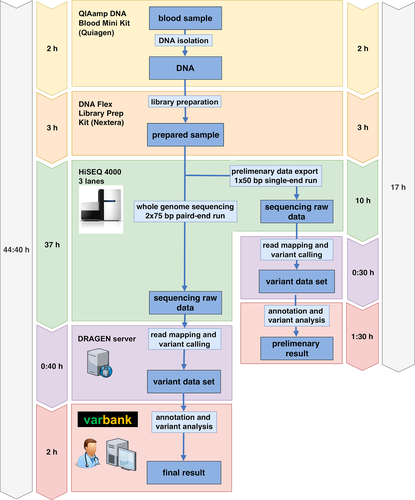
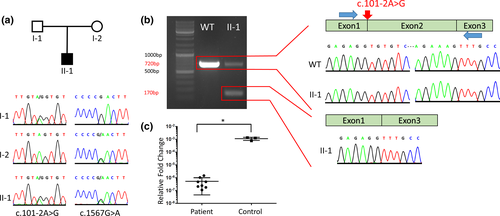
2.1 mRNA analysis
RNA from cultured patient skin fibroblasts was isolated and purified using the Qiagen RNeasy mini kit including DNase digestion, according to the kit protocol. cDNA was synthesized from 500 ng of RNA with random hexamers and SuperScript III reverse transcriptase (Thermo Fisher Scientific, Waltham, Massachusetts) according to the manufacturer’s instructions. To evaluate the effect of the c.101-2A>G variant on splicing, RT-PCR was performed with a pair of primers spanning exon 2 (F-GCCTCCGCTCAGTATTTGTAGC, R- CAGTCCTGGAAGTGGTAGTACG) (Figure 2). PCR products were separated by agarose gel electrophoresis, extracted from gel with NucleoSpin PCR-cleanup Kit (Macherey-Nagel, Dueren, Germany) and subsequently Sanger sequenced. In addition, expression profiling via RTq-PCR was performed using the primers specific to exon 12–13 (F-GCTGATGACATTGTTGGCCC, R-CTGTAGTTCCCCGGTGACAG) of the INSR gene. GAPDH was used as a housekeeping gene. The qPCR reactions were carried out via GoTaq qPCR Master Mix (Promega, Fichtburg, Wisconsin) in duplicated reactions. Additionally, we used Log10 serial dilutions as an internal control of our PCR reactions. The relative fold change of the samples was calculated with the 2-ΔΔCT-method. The statistical comparison between control and patient fibroblast INSR expression was performed via Mann–Whitney U test.
3 RESULTS
3.1 Case presentation
Our patient was the second child of nonconsanguineous parents. At 25 weeks of gestation intrauterine growth retardation (IUGR) was diagnosed; the ultrasound was otherwise unremarkable. No prenatal genetic testing had been performed. Due to progressive IUGR, the male infant was delivered via Caesarean section at 31 + 5 weeks of gestation (birth weight: 752 g [<third centile], body length: 35 cm [<third centile], head circumference: 26,5 cm [third centile]). The neonate adapted well after delivery (APGAR 4/7/8) and was admitted to the NICU with spontaneous breathing under noninvasive ventilation (DuoPAP). Weaning from respiratory support was successful after 11 days. Massive hypertrophic cardiomyopathy with significant LVOT obstruction was diagnosed on routine postnatal echocardiography and treatment with bisoprolol was initiated. The male infant developed hypo-regenerative anemia, which was treated with blood transfusions and consecutive EPO and Ferrum supplementation. L-Thyroxin supplementation was started after routine testing revealed hypothyroidism (fT3: 1.4 ng/L, fT4: 6.0 ng/L, TSH: 1.02 mU/L). From the second week of life, incomplete renal Fanconi syndrome developed, resulting in sodium, potassium, calcium and chloride wasting, leading to hyponatraemia and hypokalaemia without alkalosis and renal excretion of amino acids. Ultrasound of head and abdomen were normal, apart from a nephrocalcinosis type 2b without other alterations of the urinary tract. Oral feeding was able to be successfully started over the first 2 weeks of life. Despite this, several attempts to reduce parenteral electrolyte and glucose substitution failed because of dehydration and unstable blood glucose levels as well as hyponatremia and hypokalaemia.
Apart from low carnitine, extensive metabolic investigations including German Newborn Screening (http://www.screening-dgns.de) and a karyotype did not identify the cause of the underlying disease. Thus, at the age of 2 months, our genetics laboratory was contacted by the neonatal intensive care unit and after counseling and parental consent, ultra-rapid whole genome sequencing was performed (Figure 1). This led to the identification of two compound heterozygous pathogenic variants in the gene encoding the insulin receptor. Consecutive laboratory testing revealed hyperinsulinism (Insulin: >1,000 mU/L, C-peptide: 33.6 μg/L) and low levels of insulin like growth factor 1 (IGF-1:6 μg/L [74–316]) in accordance with the genetic diagnosis of an insulin receptor defect.
After diagnosis, continuous nasogastric tube feeding with breast milk led to rapid stabilization and the patient could be discharged to ambulatory care at the age of 14 weeks. Metformin therapy (25 mg/kg/d) did not show a significant effect on blood glucoses or insulin level.
At the age of 6 months, the boy presented with gross haematuria, haematochezia and skin bleeding. Lab results showed a massively reduced INR (<0.1), supposedly as a result of liver failure. After fresh plasma administration and substitution of vitamin K INR normalized and the bleeding symptoms were successfully stopped. On clinical follow-up, the boy was admitted to hospital two times due to episodes of dehydration, electrolyte alterations and hypoglycaemia in the course of respiratory infections. Unfortunately, he passed away at the age of 8 months due to a severe bronchopneumonia.
3.2 Ultra-rapid whole genome sequencing
Sequencing reads were mapped to the hg19 human reference genome and subsequent analysis was restricted to the target regions of the Agilent SureSelect Human All Exon V6 exome sequencing kit to focus on coding and splice site variants.
The firstly exported 50 bp single-end data amounted to 328 million mapped unique sequences yielding a mean coverage of 8 and 10x coverage for 43% of the target sequences, whereas the final paired-end data led to a significantly higher mean coverage of 34, and 10x coverage for 90.2% of target regions. Even with data from the first single-end analysis we were able to diagnose Donohue Syndrome after 17 h. The full rapid-sequencing protocol, take approximately 45 h, would also have provided a very efficient diagnosis.
After quality filtering, the first single-end dataset resulted in 20 rare single nucleotide variants, which were further evaluated by the investigators. This led to the identification of two novel compound heterozygous variants in the insulin receptor gene (INSR, OMIM: 147670, RefSeq NM_000208.2) (Figure 2). The first variant was a novel missense variant in Exon 7 (c.1567G>A; p.D523N). The second variant was a novel essential splice-site variant in the first intron (c.101-2A>G) leading to skipping of exon 2 (Figure 2(a),(b)). Both variants had not been reported in any database and were predicted to be deleterious by multiple in silico tools (SIFT, PolyPhen, CADD). They were classified as pathogenic (c.101-2A>G: PVS1, PM2, PM3, PP2, PP3) and likely pathogenic (c.1567G>A: PM2, PM3, PP2, PP3) according to the ACMG classification, respectively. The ESHG score classified both variants as pathogenic (c.101-2A>G: ESHG score 5 + 5; c.1567G>A: ESHG score 4 + 5). The variants were confirmed by Sanger Sequencing, and shown to co-segregate in the parents in a compound heterozygous pattern. We further performed functional work-up with qPCR analysis for the INSR gene showing a significant decrease in the mRNA expression level comparing the controls (p = .01) (Figure 2(c)). Of note, the qPCR analysis was not required for the definitive diagnosis.
4 DISCUSSION
Recently, several rapid NGS (rNGS) pipelines have been published and evaluated in larger cohorts focusing on technical and computational optimization (Brunelli et al., 2019; Clark et al., 2019; Elliott et al., 2019; Kingsmore et al., 2019; Petrikin et al., 2018; Stark et al., 2018). Stannenheim et al. introduced the idea of pulsed whole genome sequence data analysis, but this innovative idea has not been implemented in recent rNGS protocols (Stranneheim et al., 2014). So far, the fastest time to NGS aided diagnosis was reported by Clark et al. using the newest generation of NovaSeq 6,000 sequencers and automated variant analysis, reaching a median time to genetic diagnosis of 20 h and 10 min (Clark et al., 2019). Here, we present an ultra-rapid WGS pipeline based on the analysis of preliminary data from an early stage of the sequencing process and successfully applied it in a critical preterm infant as an individual pilot. We show that skilled manual variant filtering by clinical experts after state-of-the-art variant annotation may lead even in low coverage data to a clinically convincing preliminary diagnosis, which was achieved after 17 h for the patient described here. In combination with the newest generation of sequencing techniques and automated variant analysis, as described recently by the group of Kingsmore et al., our approach has the potential to provide genetic diagnosis within a few hours after collection of the sample (Clark et al., 2019; Kingsmore et al., 2019). Although there are some limitations of our ultra-rapid protocol, since other genes might not be sufficiently covered after the first 50 bp sequencing. We mention here that our full rapid-sequencing protocol would provide a state-of-art covered genome within 45 h only.
Homozygous or compound-heterozygous mutations of the insulin receptor (INSR) gene cause insulin resistance leading to a broad clinical spectrum. Donohue syndrome (1 per 4,000,000 births) represents the most severe phenotype related to this gene. Patients present with intra- and extrauterine growth retardation, fasting hypoglycaemia and postprandial hyperglycaemia (Kirkwood, Stuart, & Harding, 2018). Additional features are dysmorphic facial features, acanthosis nigricans, decreased subcutaneous fat, muscle wasting, cardiomyopathy and nephrocalcinosis (Kirkwood et al., 2018; Simpkin et al., 2014). Our patient presented with a few of the classical features of Donohue syndrome like growth retardation, low subcutaneous fat and alterations of blood glucose and electrolytes. Despite this, he could not be diagnosed clinically in time even by experienced neonatologists and pediatricians as well as clinical geneticists. Cases like this are experienced by many clinicians even in tertiary care or university hospitals because these clinical features and problems are difficult to distinguish from common complications in preterm newborns. Other typical features like hypertrichosis or acanthosis nigricans were not present in our patient. The fact that our ultra-rapid emergency genome pipeline for rare disorders provided the diagnosis within 17 h shows again the power of rNGS in severely ill newborns, because the disease phenotypes are often not completely developed or can be masked by common issues of term and especially preterm neonates (Farnaes et al., 2018; Kingsmore et al., 2019; Pettinger et al., 2019).
Treatment options are very limited in Donohue syndrome; nonetheless, early diagnosis guides clinicians in counseling of parents and establishing palliative care according to their needs. In general, stable blood glucoses can be achieved by continuous enteral nutrition or parenteral glucose therapy (Kirel et al., 2017; Semple, Williams, & Dunger, 2010). Metformin has been described having positive effects on blood glucose and hyperinsulinism in patients with INSR variants leading to a less severe phenotype, while the effect in Donohue syndrome is unclear (Kirel et al., 2017; Semple et al., 2010). In our patient, stable blood glucoses and transfer to ambulatory care, which was the main desire of the family, was achieved by continuous nasogastric feeding. Additional Metformin therapy did not show any effect on blood glucose or insulin level. On follow-up, our patient presented with reduced INR and hemorrhage, supposedly as a result of liver failure in the course of the main disease. The relation of these findings to a potential side effect of Metformin was considered, and the therapy terminated. There are only a small number of case studies available on IGF-1 therapy in patients with Donohue syndrome, and these do not provide evidence for a conclusive effect on survival (Hovnik et al., 2013; Kirel et al., 2017; Kirkwood et al., 2018). Most cases of Donohue syndrome harbor two loss of function variants in the insulin receptor. Our RNA analysis could prove the truncating effect of the splice variant but also that remained expression of the normal length transcript harboring the missense variant. Thus, our patient might have had a minimal remaining function of the insulin receptor and in retrospect, a therapeutic trial with high doses of insulin analogs could have had an additional benefit for the patient. In our particular case, the parents decided against a therapeutic trial.
Several studies have shown a positive impact of early genomic diagnosis on patient counseling and treatment outcomes (Farnaes et al., 2018; Gubbels et al., 2020; Lunke et al., 2020; Meng et al., 2017). Timely genetic diagnoses have led to management changes including shift to palliative care, medication changes, involvement of additional specialties, and the consideration of new experimental therapies (Gubbels et al., 2020; Lunke et al., 2020). Furthermore, by improving care and avoiding unnecessary diagnostics, procedures and medication, rNGS in the NICU has been shown to be cost-effective and even to reduce the health care costs per patient (Farnaes et al., 2018; Stark et al., 2018). Despite all the proven positive impact, with the recent breakthroughs in gene therapy of rare genetic disease as well as enzyme substitution in congenital metabolic diseases, the optimal benefit from rapid NGS diagnostics requires further minimization of time from onset of symptoms to the initiation of an effective treatment. In these situations, even a few hours could make the decisive difference, as illustrated by a recent case of molybdenum cofactor deficiency reported by Kingsmore et al.(Kingsmore et al., 2020). In our case, the genetic diagnosis had a direct impact on clinical care leading to rapid transfer to ambulatory care. An earlier diagnosis would additionally have saved several weeks of intensive care treatment and parenteral nutrition.
While most published studies focused on technical improvements of the sequencing pipeline and variant interpretation, Lunke et al. tested the clinical feasibility of ultra-rapid NGS in critically ill infants in the Australian Public Health Care System. Under these conditions they reported a mean time from sample receipt to ultra-rapid exome sequencing report of 3.3 days (Lunke et al., 2020). Time from hospital admission to ultra-rapid exome sequencing report was 17.5 days, highlighting practical complications such as delay of referral to genetic counseling, availability of parents for genetic counseling and consent and sample transportation time. To further minimize the time from indication and initiation of exome or genome sequencing to the obtaining of the results for clinical action, one should aim to implement the ultra-rapid NGS as routine procedure in the tertiary care NICUs, as demonstrated in our proof-of-principle case. At the moment this will only be possible in developed countries with a public funded health care system. But as costs are expected to decrease with further technical progress and implementation into routine testing, in a second phase also smaller hospitals and developing countries might be able to establish emergency sequencing diagnostics. Here, we offer a protocol, with the first read-out within 17–18 h and a full read-out of the genome within 45 h (below 2d). The implementation into health care systems will only be successful if clinicians experience ultra-rapid NGS as useful in their daily clinical work, with direct impact on patient treatment decisions and demand it from the payors and others who can fund this type of work. To support optimal outcomes and experiences, the sequencing technology should be as fast as possible to provide results as soon as possible. In this context, our ultra-rapid NGS pipeline will expand the impact and utility by further minimizing sequencing time, and provides a powerful approach based on the currently available sequencing technologies.
ACKNOWLEDGMENT
We are grateful to the patient and his family for participating in our pilot study. We would like to thank Dr. Mirko Rehberg and Prof. Eckhard Schönau for the ambulatory clinical care of our patient. We thank the Regional Computing Center of the University of Cologne (RRZK) for providing computing time for data analyses on the DFG-funded High-Performance Computing (HPC) system CHEOPS as well as technical support. Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Daniel Bamborschke and Sebahattin Cirak wrote the paper. Sebahattin Cirak, Susanne Motameny, Holger Thiele, Janine Altmüller and Peter Nürnberg developed and executed the NGS pipeline. Daniel Bamborschke, Özkan Özdemir and Mona Kreutzer performed the genetic analysis of the patient and the Sanger sequencing and qPCR experiments. Angela Kribs and Jörg Dötsch recruited the patient and provided clinical data. All authors reviewed the paper and contributed to scientific content.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from th corresponding author upon reasonable request.




