Grange syndrome due to homozygous YY1AP1 missense rare variants
Funding information: John Ritter Research Foundation; National Heart, Lung, and Blood Institute, Grant/Award Numbers: P01HL110869-01, RO1 HL62594; Texas Heart Institute Fibromuscular Dysplasia Project
Abstract
Grange syndrome (OMIM 602531) is an autosomal recessive condition characterized by severe early onset vascular occlusive disease and variable penetrance of brachydactyly, syndactyly, bone fragility, and learning disabilities. Grange syndrome is caused by homozygous or compound heterozygous loss-of-function variants in the YYA1P1 gene. We report on the case of a 53-year old female with novel homozygous missense variants in YYA1P1 (c.1079C>T, p.Pro360Leu), presenting with a history of brachysyndactyly, hypertension, and ischemic stroke. Imaging studies revealed stenosis of the bilateral internal carotid with extensive collateralization of cerebral vessels in a moyamoya-like pattern, along with stenosis in the splenic, common hepatic, celiac, left renal, and superior mesenteric arteries. Functional studies conducted with the patient's dermal fibroblasts suggest that the p.Pro360Leu variant decreases the stability of the YY1AP1 protein. This is the first report of a missense variant associated with Grange syndrome characterized by later onset of vascular disease and a lack of developmental delay and bone fragility.
1 INTRODUCTION
Grange syndrome (OMIM 602531) is an autosomal recessive condition with severe and early-onset vascular disease characterized by stenosis or occlusion of arteries, primarily the renal, cerebral and abdominal arteries, along with variable penetrance of brachydactyly, syndactyly, bone fragility, and learning disabilities. After its first report in four members of a family from the United States (Grange et al., 1998), four additional unrelated cases were subsequently reported (Volonghi et al., 2012; Wallerstein et al., 2006; Weymann et al., 2001). The etiology of Grange syndrome is due to homozygous loss-of-function rare variants in YY1AP1, which encodes a component of the INO80 chromatin-remodeling complex (Guo et al., 2017). We report here a patient with vascular disease consistent with Grange syndrome and a rare homozygous missense variant in YY1AP1 (c.1079C>T, p.Pro360Leu), and demonstrate that this variant leads to YY1AP1 instability.
2 MATERIAL AND METHODS
2.1 Patient material and clinical investigation
Medical records, computed tomographic (CT) images, and DNA samples were collected from the proband and her relatives after informed consent was obtained. Approval for this study was provided by the Institutional Review Board at the University of Texas Health Science Center at Houston.
2.2 Sequencing/bioinformatics analysis
Bidirectional Sanger DNA sequencing of the YY1AP1 gene was performed on DNA fragments amplified with intron-based, exon-specific primers from genomic DNA. Polymerase chain reaction (PCR) amplifications were carried out using HotStarTaq Plus DNA polymerase (Qiagen Inc. Valencia, CA). PCR products were treated with ExoSAP-IT (Affymetrix, Inc., OH) to digest excess primers and followed with sequencing using the BigDye sequencing reaction mix (Applied Biosystems, CA). The sequencing PCR products were purified using the BigDye XTerminator kit (Applied Biosystems, CA) and then loaded on an ABI3730xl sequencing instrument using the Rapid36 run module. DNA sequencing results were analyzed using the Mutation Surveyor software (SoftGenetics, PA). Annotation of variants identified in the proband was performed using the dbNSFP3.5a database. The c.1079C>T, p.Pro360Leu variant in the YY1AP1 gene was called based on the transcript numbers Ensembl: ENST00000368339; NCBI: NM_001198903.1.
2.3 Cell culture experiments
Dermal fibroblasts were explanted from the proband and matched with control fibroblasts for age and gender. The fibroblasts were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (HyClone, Logan, UT), containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100ug/ml streptomycin and 250 ng/ml amphotericin at 37°C. For immunoblot analyses and cycloheximide chase experiments, cells were seeded at 70 cells/mm2 in a 6 cm tissue culture dish. Cycloheximide (100ug/mL; Millipore-Sigma) was added to the medium for the indicated time. Cell lysates were harvested, subjected to SDS-PAGE analysis, and probed with anti-YY1AP1 (Millipore-Sigma ABC477) or anti-GAPDH (Cell Signaling Technologies 2,118) antibodies.
3 RESULTS
3.1 Clinical report
The proband is a 53-year-old woman with deep-set eyes, thin lips, and normal height and intellect. She was born with cutaneous syndactyly of the third and fourth digits of the left hand, requiring surgery, as well as bilateral syndactyly of the second and third digits of the feet. She has small fingers with slightly bulbous fingertips, mild scoliosis and pes planus. Her skin is slightly mottled, consistent with livedo reticularis. She is the youngest of seven siblings born to consanguineous parents (second cousins) of mixed English and French ancestry (Figure 1a). An unaffected sister and a brother with a history of cerebral aneurysms underwent genetic testing. Another sister with long-standing hypertension and vaso-occlusive disease declined genetic testing. The proband's four additional siblings do not have hypertension or vascular disease and declined testing. Her father had late onset coronary artery disease and died at the age of 90 years. Her mother had a history of atrial fibrillation, migraines, livedo reticularis, and macular degeneration, and died of a stroke at the 88 years of age.
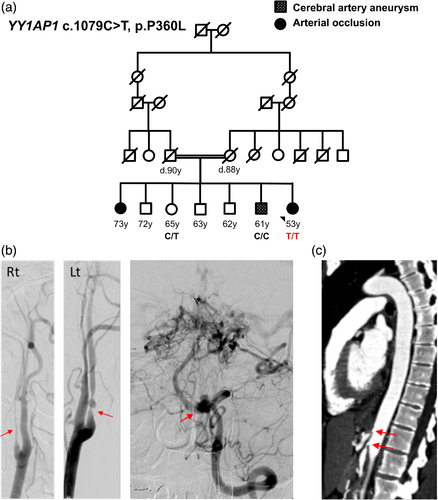
The patient had migraines starting at 12 years of age. She was diagnosed with hypertension at 25 years of age, which is currently managed with an angiotensin converting enzyme inhibitor and a calcium channel blocker. She takes thyroid hormone therapy for hypothyroidism, diagnosed at the age of 38 years. She had reproductive problems, which included seven miscarriages and one ectopic pregnancy. She had a son at the age of 23 years but was unable to conceive another viable pregnancy.
At the age of 42 years, the patient presented with symptoms of an ischemic stroke. Imaging revealed a small left occipital infarct, bilateral stenosis of the internal carotid arteries, multiple intracranial aneurysms, and tortuosity of the anterior and middle cerebral arteries and left vertebral artery. She underwent a left carotid artery endarterectomy and angioplasty upon admission during this episode. Imaging of her head and neck has remained unchanged with the most recent imaging studies at the age of 52 years showing significant bilateral stenosis of the internal carotid arteries (Figure 1b), a 7 mm lobulated aneurysm arising from the medial aspect of the left vertebral artery, and extensive collateralization in a moyamoya-like pattern (Figure 1c). Her cerebrovascular disease is being managed with antiplatelet therapy and blood pressure control.
Further imaging of her abdomen and pelvis at the age of 42 years revealed stenosis of the splenic, common hepatic, celiac, and superior mesenteric arteries (Figure 1d). She underwent a mesenteric artery bypass surgery and angioplasty of the superior mesenteric artery. In retrospect, these vascular occlusions may be the etiology the proband's long-standing gastrointestinal symptoms, including abdominal pain and alternating constipation and diarrhea. At 51 years of age, cardiac workup revealed tortuosity of coronary arteries. Imaging studies at the age of 52 years of age showed severe stenosis of the left renal artery, along with an atrophic left kidney and low-density lesions in the liver and kidneys. Genetic testing was negative for pathogenic variants in TGFBR1, TGFBR2, SLCA10, ACTA2, and COL3A1.
At 51 years of age, the diagnosis of Grange syndrome was raised and Sanger sequencing of YY1AP1 showed a novel homozygous missense pathogenic variant (c.1079C > T, p.Pro360Leu). This variant is absent in both the gnomAD v2.1.1 and ExAC databases and has a CADD score of 25. The p.Pro360 residue is conserved with a phastCons100way_vertebrate score of 1 (this analysis scores each residue for conservation on a scale between 0 and 1; 1 indicates the highest level of conservation).
After identification of the homozygous YY1AP1 variant in the patient, DNA samples were acquired from the patient's siblings for genetic testing. The unaffected sister is heterozygous for this YY1AP1 variant (Figure 1a). The brother, who does not carry the variant, has a history of hypertension and dyslipidemia and was diagnosed with 1 mm basilar aneurysm and a 6 mm left posterior inferior cerebellar aneurysm diagnosed at the age of 54 years. Review of his CT brain angiograms showed that his cerebrovascular findings were distinct from the vascular disease described in the proband, specifically no evidence of occlusion of the arteries.
3.2 Functional studies
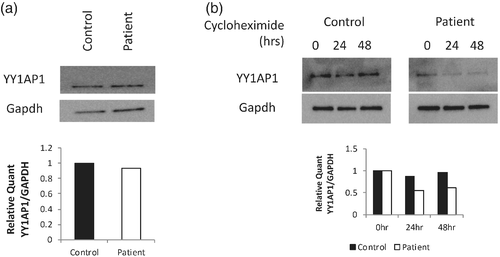
To study the mutant YY1AP1 proteins, dermal fibroblasts were explanted from the affected proband and compared with cells from an age- and gender-matched unaffected individual. At baseline, YY1AP1 protein levels are similar between the two cell lines (Figure 2a) and the protein was localized to the nucleus of the cell in both the patient's and control cells (data not shown). The protein translation inhibitor, cycloheximide, was used to assess whether the p.Pro360Leu variant decreased the stability of the YY1AP1 protein (Kwartler et al., 2014). After 24 hours of cycloheximide treatment, YY1AP1 protein levels are significantly decreased in the fibroblasts from the patient, while the control cells maintain high levels of YY1AP1 protein during the 24 hours (Figure 2b). These results suggest that the p.Pro360Leu variant decreases the stability of the YY1AP1 protein.
4 DISCUSSION
We describe here the first case of Grange syndrome due to a YY1AP1 homozygous missense pathogenic variant. The proband presented with typical vascular complications of Grange syndrome, including cerebral, renal, and mesenteric artery stenosis and syndactyly of the hands and feet. The vascular disease is later onset when compared to previously reported Grange syndrome cases; affected individuals present with bilateral renal stenosis with secondary hypertension as early as 4 months of age and up to 18 years of age (Table 1). In contrast, the patient presented here had onset of hypertension at 25 years of age and unilateral renal artery stenosis diagnosed at 52 years of age. In addition, vaso-occlusive events leading to serious complications of cerebral or mesenteric ischemia were diagnosed between 15 and 26 years of age in previously reported Grange syndrome cases, whereas the patient reported here initially presented with complications of vascular disease at the age of 42 years. Grange syndrome features with variable penetrance, for example, developmental delay and bone fragility, are not present in our patient, which may also be indicative of a milder phenotype. Livedo reticularis and arterial tortuosity are features observed in this patient that have not been previously described in Grange syndrome cases. The arterial tortuosity could be a late manifestation of Grange syndrome; the patient reported here is the oldest case reported to date. Alternatively, the observed cerebral arterial tortuosity may be secondary to occlusion of the internal carotids and development of collaterals (Choy et al., 2006) and coronary artery tortuosity can be associated with increased age and sustained hypertension (Chiha et al., 2016, Han, 2012).
| Patient 1 (Grange te al., 1998) | Patient 2 (Grange et al., 1998) | Patient 3 (Grange et al., 1998) | Patient 4 (Grange et al., 1998) | Patient 5 (Weymann et al., 2001) | Patient 6 (Wallerstein et al., 2006) | Patient 7 (Volonghi et al., 2012) | Patient 8 (Guo et al., 2017) | This case | |
|---|---|---|---|---|---|---|---|---|---|
| YY1AP1 pathogenic variants | Compound heterozygous (Q242X, L797X) | Compound heterozygous (Q242X, L797X) | Not sequenced | Not sequenced | Homozygous (E801X) | Homozygous (E636Pfs*13) | Not sequenced | Homozygous (Q222X) | Homozygous (P360L) |
| Gender | Female | Male | Female | Female | Male | Female | Female | Female | Female |
| Ischemic event | Cerebrovascular event (TIA) | Mesenteric ischemia (weight loss, post prandial abdominal pain) | Myocardial ischemia (chest pain, sudden death) | Cerebrovascular event (TIA, right hemiparesis) | Cerebrovascular event (subarachnoidal hemorrhage) | None | Cerebrovascular event (subarachnoidal hemorrhage) | None | Cerebrovascular event (stroke) |
| Age of ischemic event | 26 years | 27 years | 18 years | 10 years | 15 years | NA | 18 years | NA | 42 years |
| Skeletal abnormalities | |||||||||
| Bone fragility | + | + | + | + | − | + | − | − | − |
| Brachydactyly | + | + | + | + | + | + | + | + | + |
| Syndactyly | + | + | − | − | + | + | + | − | + |
| Arteriopathies | |||||||||
| Cerebral arteries stenosis | + | + | Unknown | + | + | + | + | − | + |
| Intracranial aneurysms | − | − | Unknown | + | + | − | + | − | + |
| Cerebral collateral vessel formation | − | + | Unknown | + | + | + | + | − | + |
| Coronary arteries stenosis | − | − | + | + | − | − | − | − | − |
| Abdominal arteries stenosis | + | + | Unknown | + | + | − | + | − | + |
| Renal arteries stenosis | Bilateral | Bilateral | Unilateral | Bilateral | Bilateral | Bilateral | Bilateral | − | Unilateral |
| Chronic hypertension (age of diagnosis) | + | + | + (5 years) | + (3 years) | + (15 years) | + (4 months) | + (18 years) | − | + (25 years) |
| Arterial tortuosity | − | − | − | − | − | − | − | − | + |
| Developmental delays | − | + | + | + | + | + | + | + | − |
All previous reports of pathogenic variants in YY1AP1 have been unambiguous loss-of-function alleles that eliminate protein production. In contrast, this missense variant did not affect steady state protein levels or localization to the nucleus, but the mutant protein was degraded more rapidly compared with wild type YY1AP1. In smooth muscle cells, YY1AP1 protein levels increase transiently in response to transforming growth factor β1 (TGFβ1), which drives differentiation to a more contractile phenotype (Guo et al., 2017). The less stable p.Pro360Leu variant protein may alter the dynamics of YY1AP1 expression in response to stimuli like TGFβ1, which has the potential to impact the phenotype of the smooth muscle cells and contribute to the occlusive vascular disease seen in this patient.
There are no reports in the literature of women with Grange syndrome who have attempted to get pregnant or successfully reproduced. The patient reported here was successful in conceiving, but had difficulties carrying the pregnancy to term. Her multiple miscarriages could be indicative of vascular occlusive lesions in the placenta due to the underlying YY1AP1 pathogenic variant, disrupting blood flow to the fetus. The inability to carry a full-term pregnancy is another potential clinical complication that should further be assessed in other women with Grange syndrome.
ACKNOWLEDGMENTS
The authors thank the patients for their participation in this study. The following provided funding for this study: National Institutes of Health (RO1 HL62594 and P01HL110869-01), and the John Ritter Research Foundation.
CONFLICT OF INTEREST
None of the authors have any conflicts to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports this study is available from the corresponding author on reasonable request.




