IFT172 as the 19th gene causative of oral-facial-digital syndrome
Funding information: Initiative on Rare and Undiagnosed Diseases, Grant/Award Number: JP17ek0109151; JSPS KAKENHI, Grant-in-Aid for Early-Career Scientists, Grant/Award Number: JP19K17342
Ciliopathies are a group of disorders resulting from either the abnormal formation or function of cilia and are associated with alterations in more than 150 genes coding for ciliary proteins (Reiter & Leroux, 2017). Ciliary proteins constitute motile and non-motile primary cilia and play biologic roles in various organs including the retina, central nervous system, olfactory epithelium, heart, liver, kidney, skeletal system, gonads, and adipose tissue. As a reflection of these diverse roles in various organs, broad clinical features have been described.
Ciliopathy disorders are classified into subgroups depending on their particular combination of clinical features. Several classic congenital malformation syndromes have been recognized as prototypic ciliopathies. For example, Joubert syndrome (JBTS) is characterized by congenital brain defects including cerebellar vermis hypoplasia and intellectual disability; Senior–Loken syndrome (SLS) is characterized by nephronophthisis, retinal degeneration, and coloboma; and Bardet–Biedl syndrome (BBS) is characterized by obesity, intellectual disability, retinal degeneration, cystic kidney disease, post-axial polydactyly, hypogonadisms. Short-rib thoracic dysplasia (SRTD) and oral-facial-digital (OFD) syndrome are also classified as ciliopathies (Braun & Hildebrandt, 2017).
OFD syndrome is characterized by very specific and almost pathognomonic malformation in the oral cavity including multiple frenula, hamartoma of the tongue, and digital defects (Bruel et al., 2017). Historically, OFD syndrome has been classified into 13 subtypes (types 1–13) based on clinical features in addition to the core features including oral defects. Five subtypes (types 14–18) have been proposed based on causative genes including C2CD3, KIAA0753, TMEM107, INTU, and IFT57. Recently, causative variants in four more genes (i.e., C5orf42, TMEM138, TMEM231, and WDPCP) have been reported. Alterations in yet unknown genes encoding ciliary proteins may also be associated with OFD (Bruel et al., 2017).
Recently, ciliopathy-related disorders have been ascribed to biallelic variants in IFT172, which belongs to the IFT family the members of which play critical roles in intraflagellar transport (Bujakowska et al., 2015; Halbritter et al., 2013). Germline variants in IFT172 have been observed in patients with the clinical features of JBTS, BBS, isolated retinal degeneration, and SRTD, but not in those with OFD-like features (Bujakowska et al., 2015; Halbritter et al., 2013; Schaefer et al., 2016). Here, we report a male patient with the core features of OFD who had compound heterozygous variants in IFT172. Our observation expands the phenotypic spectrum of IFT172-related disorders.
The patient was a 3-year-old boy who had been born at 40 and 3/7 weeks of gestation to nonconsanguineous parents with no significant family medical history. A neonatal echography showed congenital heart diseases. His birth weight was 3,102 g (−0.1 SD), his length was 49.0 cm (−0.4 SD), and his head circumference was 36.4 cm (+2.3 SD). His Apgar scores were 8 and 8 at 1 and 5 min. He had postaxial polydactyly of the hands and feet, which had been repaired at 15 and 18 months (Figure 1), and congenital heart diseases (a single atrium, an atrioventricular septal defect, and a persistent left superior vena cava, repaired at 1 year of age).
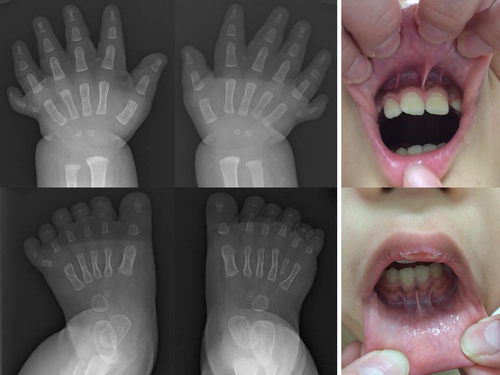
At the age of 3 years, the patient had frontal bossing, a flat face and brachymetaphalangy, according to the metacarpophalangeal profiling based on ethnically-matched control data (Matsuura & Kajii, 1989). Brachymetaphalangy is a known feature of Ellis–van Crevelt syndrome, which is a prototypic skeletal dysplasia included in the ciliopathies. He had difficulties in feeding and received nutrition via a nasogastric tube for 3 weeks. He had developmental delays; he gained eye tracking at the age of 7 months, head control at the age of 9 months, was able to walk while holding on to something at the age of 26 months and spoke meaningful words at the age of 3 years. At the age of 3 years and 1 month, his weight was 12.9 kg (−1.0 SD), his length was 87.0 cm (−2.0 SD) and his head circumference was 49.0 cm (−0.15 SD). The patient was of a short stature with delayed bone age and a relatively low somatomedin level for his age. Ultrasound evaluation of the liver and kidneys revealed no abnormalities. Fundoscopic examination showed no retinal lesions. MRI of the brain was normal. Dysmorphic features included a bifid tongue, multiple and hypertrophied oral frenula (Figure 1), and a missing left incisor.
A trio exome analysis of the genomic DNA derived from the peripheral blood of the patient and his parents showed compound heterozygous variants of the IFT172 gene: Chr2(GRCh37):g.27712477C>T, c.39+5G>A and Chr2(GRCh37):g.27695163A>C, c.1478T>G (NM_01566.2). Within the family, the two variants segregated with the disease in a manner that was compatible with autosomal recessive inheritance. Both variants are extremely rare: The c.39+5G>A variant was observed in only one person in a cohort of 3,000 normal Japanese people (Tadaka et al., 2019). c.1478T>G has never been encountered in any Japanese cohort and is not included in the gnomAD database (Lek et al., 2016). Both variants are absent in the pathogenic variant databases, namely, ClinVar and the HGMD database (Landrum et al., 2014; Stenson et al., 2017).
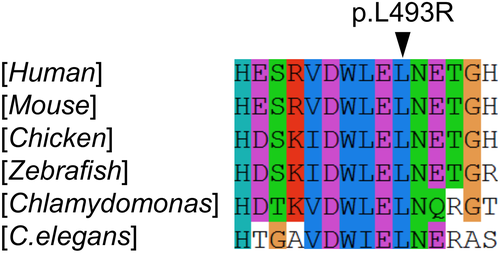
The compound heterozygous variants of the IFT172 gene are most likely to be responsible for the patient's phenotype: The variant c.39+5G>A represents a G to A substitution at position +5 of the splicing donor site of intron 1. This substitution of G to A at position +5 of the donor site which involves interactions with U1 and U6 snRNAs (Buratti et al., 2007) is now increasingly recognized as a cause of aberrant splicing (Shiraishi et al., 2018). Indeed RNA-seq of the peripheral blood indicated that only the c.1478T>G variant was observed at the transcriptome level. Since the patient was heterozygous for the c.1478T>G allele, the apparent monoallelic expression indicated that the transcript derived from the c.39+5G>A allele was subject to nonsense-mediated mRNA decay. Overall, the c.39+5G>A allele was rated as “pathogenic” according to the standards and guidelines for the interpretation of sequence variants by the American College of Medical Genetics and Genomics (Richards et al., 2015). The other missense variant, c.1478T>G (NM_015662.2), p.(Leu493Arg), was located within the eighth WD-40 repeats domain which is highly conserved according to the CLUSTALW program (Larkin et al., 2007). The amino acid residues surrounding variants VDWLELN is conserved down to Chlamydomonas reinhardtii (Figure 2). The Pathogenicity of the variant was predicted as follows: SIFT (“deleterious,” with a score of 0) (Vaser, Adusumalli, Leng, Sikic, & Ng, 2016), PROVEAN (“deleterious,” with a score of −5.6) (Choi & Chan, 2015), and PolyPhen2 (“probably damaging,” with a score of 1.0) (Adzhubei, Jordan, & Sunyaev, 2013). The combined annotation dependent depletion (CADD) score, which integrates these multiple annotations into one metric predicated a score of 29.2, which is well above the threshold of 25 and is highly indicative of deleteriousness (Kircher et al., 2014). Overall, the allele c.1478T>G was scored as “likely pathogenic” according to the standards and guidelines for the interpretation of sequence variants by the American College of Medical Genetics and Genomics (Richards et al., 2015).
Trio exome analysis detected two de novo coding variants, SPTBN5 and SHC2, neither of which was associated with any human diseases. No biallelic putatively pathogenic variants were detected in any gene other than IFT172.
We report a male patient with biallelic putatively pathogenic variants in IFT172 and a unique combination of ciliopathy-associated features including post-axial polydactyly, laterality defects of the heart tube (i.e., single atrium, an atrioventricular septal defect, and a persistent left superior vena cava), and multiple oral frenula. Multiple oral frenula are known to be almost pathognomonic for OFD (Mintz, Siegel, & Seider, 2005). We suggest that the IFT172 gene represents a 19th ciliopathy-related gene that causes the OFD phenotype.
Ciliopathy-related genes have been classified into several categories: genes required for (a) ciliogenesis, (b) ciliary compartmentalization, and (c) ciliary trafficking (Reiter & Leroux, 2017). Most of the genes reported to be responsible for OFD have been classified as genes responsible for ciliogenesis (OFD1 (OFD1), SCLT1 (OFD9), C2CD3 (OFD14), and INTU (OFD17)) or ciliary compartmentalization (OFD1, TCTN3 (OFD4), CPLANE1, C5ORF42, and TMEM216 (OFD6), KIAA0753 (OFD15), and TMEM107 (OFD16)), while IFT57 plays a role in ciliary trafficking. The recognition of IFT172, which plays a critical role in ciliary trafficking similar to that of IFT57, as a cause of OFD underscores the importance of ciliary trafficking in the pathogenesis of OFD. It is still unclear whether the clinical phenotype can be inferred from the genotype of IFT172 variants, which can lead to various ciliopathy disorders including Bardet–Biedl syndrome (BBS20), Joubert syndrome (JBTS), and short-rib thoracic dysplasia (SRTD10) (Bujakowska et al., 2015; Halbritter et al., 2013; Schaefer et al., 2016). Review of the previously reported patients with IFT172 variants did not reveal any apparent correlations between the genotype and phenotype (Supporting Information Table S1).
In conclusion, this is the first report of an IFT172 defect in an OFD patient, defining IFT172 as the 19th OFD gene. The documentation of a defect in IFT172, in addition to the known influence of IFT57, confirms the IFT pathway as a pathogenetic mechanism for OFD.
ACKNOWLEDGMENTS
We thank Ms. Chika Kanoe, Ms. Yumi Obayashi and Ms. Keiko Tsukue for their technical assistance in the preparation of this article. This work was supported by Initiative on Rare and Undiagnosed Diseases (grant number JP17ek0109151) from the Japan Agency for Medical Research and Development, and by JSPS KAKENHI, Grant-in-Aid for Early-Career Scientists (grant number JP19K17342).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Database of Pathogenic Variants at https://dpv.cmg.med.keio.ac.jp/dpv-pub/top, reference number DPVS: 10607.1 and https://dpv.cmg.med.keio.ac.jp/dpv-pub/top, reference number DPVS:10606.1.




