ADA2 deficiency in a patient with Noonan syndrome-like disorder with loose anagen hair: The co-occurrence of two rare syndromes
Abstract
Noonan syndrome-like disorder with loose anagen hair (NS/LAH) is one of the RASopathies, a group of clinically related developmental disorders caused by germline mutations in genes that encode components acting in the RAS/MAPK pathway. Among RASopathies, NS/LAH (OMIM 607721) is an extremely rare, multiple anomaly syndrome characterized by dysmorphic facial features similar to those observed in Noonan syndrome along with some distinctive ectodermal findings including easily pluckable, sparse, thin, and slow-growing hair. ADA2 deficiency (DADA2, OMIM 615688) is a monogenic autoinflammatory disorder caused by homozygous or compound heterozygous mutations in ADA2, with clinical features including recurrent fever, livedo racemosa, hepatosplenomegaly, and strokes as well as immune dysregulation. This is the first report of NS/LAH and ADA2 deficiency in the same individual. We report on a patient presenting with facial features, recurrent infections and ectodermal findings in whom both the clinical and molecular diagnoses of NS/LAH and ADA2 deficiency were established, respectively.
1 INTRODUCTION
RASopathies are a group of clinically related developmental disorders including Noonan syndrome, Noonan syndrome-like disorder with loose anagen hair (NS/LAH), Costello syndrome, cardio-facio-cutaneous (CFC) syndrome, Noonan syndrome with multiple lentigines, hereditary gingival fibromatosis and capillary malformation-arteriovenous malformation (Aoki, Niihori, Inoue, & Matsubara, 2016). Although each syndrome has several unique features, some physical and developmental findings may overlap as well. RASopathies are genetically heterogenous. They are caused by germline mutations in genes encoding RAS superfamily GTPases and functionally related proteins (KRAS, NRAS, HRAS, RIT1, RRAS, CBL, PTPN11, SOS1, SHOC2, neurofibromin, SPRED) or transducers of MAPK cascade (BRAF, RAF1, MEK1, MEK2; Garavelli et al., 2015). Recently some novel variants in RASA2, A2ML1, SOS2, LZTR1 and PPP1CB have also been reported in the etiology (Aoki et al., 2016). Among RASopathies, NS/LAH (OMIM 607721) is an extremely rare multiple anomaly syndrome characterized by facial features similar to those observed in Noonan syndrome including hypertelorism, ptosis, down-slanting palpebral fissures, low-set posteriorly angulated ears, and overfolded pinnae. Patients also have short stature, cognitive deficits, relative macrocephaly, cardiac defects, and ectodermal abnormalities of which the most characteristic feature is the hair anomaly (Cordeddu et al., 2009). Easily pluckable, thin, sparse and slow-growing hair in anagen phase devoid of sheaths is characteristic for NS/LAH (Mazzanti et al., 2013). In addition dry, hairless, usually darkly pigmented skin, hyperkeratosis and dermatitis are among the other usual ectodermal findings. To date approximately 70 patients with NS/LAH have been reported (Bertola et al., 2017; Capalbo et al., 2012; Cordeddu et al., 2009; Garavelli et al., 2015; Gripp et al., 2013; 2016; Hoban, Roberts, Demmer, Jethva, & Shephard, 2012; Komatsuzaki et al., 2010; Marchetti et al., 2016; Mazzanti et al., 2013; Zambrano et al., 2017; Zmolikova et al., 2014). Heterozygous S2G mutation in SHOC2 (OMIM 602775) is identified in majority of patients with NS/LAH (Aoki et al., 2016), nevertheless mutations in Protein Phosphatase 1 Catalytic Subunit Beta Isoform (PPP1CB, OMIM 600590), has also been reported recently (Bertola et al., 2017; Gripp et al., 2016). There are no significant reported phenotype differences between NS-LAH due to SHOC2 and PPP1CB.
DADA2 (OMIM 615688), a monogenic autoinflammatory disorder, is caused by homozygous or compound heterozygous mutations in ADA2 (OMIM 607575; Lee, 2018; Moens, Hershfield, Arts, Aksentijevich, & Meyts, 2019). DADA2 has been reported in more than 170 patients, however, the actual number of affected individuals is suspected to be higher (Moens et al., 2019). Mutations in ADA2 lead to unprovoked inflammation in various tissues, particularly in vascular system (Lee, 2018; Moens et al., 2019). Vascular inflammation is responsible for the majority of the phenotypical features including intermittent fever, rash, PAN-like symptoms and recurrent strokes as well as hematological and rheumatological features (Lee, 2018; Moens et al., 2019). The isolated autoinflammatory disease phenotype has emerged as a phenotype with multisystemic involvement affecting almost all organ systems with variable clinical severity and age of onset after the description of several patients for the last 5 years (Moens et al., 2019). Hypogammaglobulinemia and bone marrow suppression have been reported in almost half of the patients with ADA2 deficiency (Schepp et al., 2017). To our knowledge, ADA2 deficiency and NS/LAH has not previously been reported together in the same individual.
1.1 Clinical report
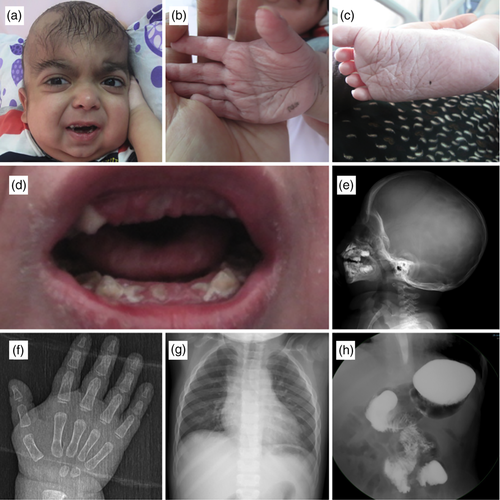
The patient was the fifth child of healthy, consanguineous (first cousins) parents with an unremarkable prenatal history. The patient was born at term with a birth weight of 3,000 g (10–25th centile). Birth length and head circumference were not noted. The patient had respiratory distress requiring mechanical ventilation and was followed in the neonatal intensive care unit 15 days. After discharge, he was hospitalized on average four times a year for recurrent pneumonia, perianal abscess, gastroenteritis, and failure to thrive. Two of these hospitalizations required admission to the intensive care unit for intractable gastroenteritis and severe pneumonia. He also suffered from recurrent eczematous skin lesions, especially in the lower extremity. He had delays in motor and mental developmental milestones; he sat without support at 17/12, walked after 35/12 and spoke a few words after 3 years of age. The patient was referred to our center at the age of 35/12 years. Physical examination revealed a body weight of 12 kg (−9.6 SD), a height of 83 cm (−3.89 SD) and a head circumference of 49 cm (−0.6 SD). He had severe growth retardation and dysmorphic facial features including prominent and wide forehead with hypertrichosis, a round face, microretrognathia, depressed nasal root, prominent eyes, down-slanting palpebral fissures with mild ptosis, arched eye brows and posteriorly angulated, low-set ears. His dental hygiene was poor, he had malocclusion and dental discoloration. In addition deep palmar and plantar creases, prominent joint laxity, thin and sparse hair were also noted (Figure 1). Echocardiography showed mitral insufficiency and mitral valve prolapse; brain magnetic resonance imaging revealed dilatation in the third and lateral ventricles, ectopic neurohypophysis and a focal cortical anomaly in the right cerebellar hemisphere (Figure 1). A choanal adhesion was noted during fiberoptic examination. Abdominal and pelvic ultrasonography, ophthalmologic and audiometric evaluations were normal. A double-contrast imaging study of upper gastrointestinal system and colonoscopy revealed malrotation of duodenum and jejenum and increased fragility of colonic mucosa, respectively. Swallowing dysfunction with risk of aspiration of liquids was demonstrated as well. Laboratory evaluation including complete blood cell count, peripheral smear, thyroid and renal function tests, blood and urine aminoacids, urine glycosaminoglycans and urinary organic acids were all normal. Immunologic evaluation revealed panhypoglobulinemia with serum levels of; IgA: 9.7 mg/dl (44–244 mg/dl), IgG: 384 mg/dl (640–2010 mg/dl), IgM: 7.9 mg/dl (52–297 mg/dl). Peripheral blood lymphocyte subsets analysis demonstrated low CD19 (1% [14–33%]) and CD4 (18% [28–47%]) levels reflecting significant reduction of mature B-cells. Lymphocyte transformation test was normal. IVIG treatment was initiated to be given every 3 weeks. However, low levels of immunoglobulin and lymphocyte subsets persisted after consecutive administration of intravenous immunoglobulin therapy. The patient was diagnosed NS/LAH after clinical evaluation. Informed consent was obtained from the parents and genetic analysis was planned accordingly.
2 MATERIALS AND METHODS
Genomic DNA samples were extracted from peripheral whole blood using the standard procedure (Taskiran et al., 2017; Utine et al., 2017). With the dysmorphic findings, Rasopathy syndromes were suspected as the primary diagnosis. Therefore, a Sanger sequencing study was carried out containing selected exons of six genes (KRAS, HRAS, MEK1, BRAF, MEK2, SHOC2) associated with this syndrome group (the list of genes and exons are provided in the Supporting Information). Sanger sequencing of the related gene regions were performed as described previously (Simsek-Kiper et al., 2013). However during the follow-up, due to the presence of atypical findings, whole exome analysis was also planned to figure out a second accompanying rare disease. Array analysis was performed to determine the coincidence of copy number variations. Microarray analysis using Agilent 8x60K v2 kit and whole exome sequencing were performed as described previously (Taskiran et al., 2017; Utine et al., 2017) as well. Identified variants of presumed pathogenic relevance were validated on the original DNA sample using conventional Sanger sequencing on an automated capillary sequencer (Applied Biosystems 3500xL Genetic Analyzer).
3 RESULTS
A de novo heterozygous, previously reported variant of SHOC2 (NM_007373) in Exon 2: c.4A>G(p.S2G) was identified in the patient. Microarray analysis was normal. In whole exome analysis, for the prioritization of variants, homozygous variants, and then noncoding and synonymous variants were filtered out. A novel homozygous missense c.578C>T(p.Pro193Leu) variation in ADA2 remained after removal of variants with high allele frequency (>1% in the ExAC data). The variant of ADA2 (c.578C>T, p.Pro193Leu) was predicted to be deleterious by SIFT (score 0.000) and PolyPhen2 (1.00). Allele frequency of the variant was 0.0000199 and was not reported in homozygous state in GnomAd. The variant in ADA2 was further verified by Sanger sequencing and segregation within family members showed that father and mother were heterozygous carriers for this alteration. The level of serum ADA2 was 1.92 U/l (<20). In our patient, oral and intravenous steroid treatments were used for vasculitis-related findings, as well as IVIG for hypogammaglobulinemia, but only a limited response could be achieved. After the establishment of DADA2 diagnosis, an anti-TNF agent with close follow-up has been started. Stem cell transplantation has also been considered among the future treatment options.
4 DISCUSSION
In this clinical report, we have described a patient with NS/LAH syndrome and ADA2 deficiency harboring SHOC2 and ADA2 mutations, respectively (Table 1). Among RASopathies, while Noonan syndrome represents the most prevalent disorder with greatest genetic heterogeneity (Aoki et al., 2016), NS/LAH remains among the less common ones. NS/LAH was reported clinically based on its distinctive hair findings in combination with growth hormone deficiency and other typical Noonan syndrome findings (Mazzanti et al., 2013), and its genetic etiology was established in 2009 (Cordeddu et al., 2009). The heterozygous S2G mutation in SHOC2, encoding a leucine-rich repeat protein implicated in fibroblast growth factor receptor signaling is responsible from the majority of patients with NS/LAH (Selfors, Schutzman, Borland, & Stern, 1998).
| NS/LAH syndrome | ADA2 deficiency | Present patient | |
|---|---|---|---|
| Facial dysmorphism | |||
| Relative macrocephaly | + | − | + |
| Wide forehead | + | − | + |
| Round face | − | − | + |
| Down-slanting palpebral fissures | + | − | + |
| Hypertelorism | + | − | + |
| Epicanthus | + | − | − |
| Hypertrophic gums | + | − | + |
| Everted lower lip | − | − | + |
| Microretrognathia | − | − | + |
| Developmental delay | + | + | + |
| Failure to thrive | + | + | + |
| Joint laxity | + | − | + |
| Ectodermal abnormalities | |||
| Deep palmar and plantar creases | + | − | + |
| Skin elasticity | + | − | + |
| Eczema | + | + | + |
| Ichthyosis | + | − | − |
| Sparse and thin hair | + | − | + |
| Rash | − | + | + |
| Intermittent fevers | − | + | + |
| Musculoskeletal involvement (myalgia/arhralgia/arhritis/myositis) | − | + | − |
| Vasculitis | − | + | + |
| Hematologic disorders (lymphopenia/neutropenia/thrombocytopenia/pancytopenia) | + | + | − |
| Malrotation of intestines | − | − | + |
| Ectopic hypophysis | − | − | + |
| Immune deficiency | − | + | + |
- Abbreviation: NS/LAH, Noonan syndrome-like disorder with loose anagen hair.
RASopathies share many clinical features, including facial dysmorphism, congenital heart defects, postnatal growth failure, variable degrees of neurocognitive impairment, and skeletal as well as ectodermal anomalies and increased tumor risk (Tartaglia, Gelb, & Zenker, 2011). Ectodermal findings may particularly be helpful in the differential diagnosis of Costello, CFC, and NS/LAH syndromes. The facial features along with characteristic ectodermal findings including dry and sparse hair, darkly pigmented skin with features of dermatitis and hypertrichosis, and deep palmar and plantar creases were very suggestive of NS/LAH syndrome in the present patient. However, the finding of frequent infections was quite unusual for NS/LAH. Although hematologic findings including several different coagulation defects (factor V, VIII, XI, XII, protein C) alone or in combination and some myeloproliferative disorders including acute lymphoblastic anemia or chronic myelomonocytic leukemia may be seen in patients with RASopathies, immunodeficiency has not been reported as a consistent finding (Aoki et al., 2016). The findings of coagulopathy, thrombocytopenia and bleeding dyscrasia have been reported in patients with the recurrent S2G mutation in SHOC2 (Hoban et al., 2012). The present patient did not have any hematologic findings, instead, he displayed severe recurrent infections involving different organ systems, and laboratory findings compatible with variable immune deficiency. Isolated ventriculomegaly, posterior fossa anomalies, cerebral atrophy, hypoplastic corpus callosum, delayed gyrification of the cerebral cortex were the reported brain imaging findings among RASopathy patients (Cizmeci et al., 2018; Gripp et al., 2013; Nystrom et al., 2009). In our patient's brain imaging findings, although the third and lateral ventricles dilatation and focal cortical anomaly in the right cerebellar hemisphere were reported previously, ectopic neurohypophysis was a novel finding. Autoimmune disorders including systemic lupus erythematosus (SLE) and erythema nodosum have been reported among patients among RASopathies, especially in SHOC2 pathogenic variants (Alanay, Balci, & Ozen, 2004; Okazaki et al., 2019; Simsek-Kiper et al., 2013; Uehara, Hosogaya, Matsuo, & Kosaki, 2018). However, although the underlying mechanism cannot be fully explained, it is suggested that this coexistence of such autoinflammatory diseases with RAsopathy in multiple patients is not random (Okazaki et al., 2019; Uehara et al., 2018).
DADA2 is associated with homozygous mutations in the ADA2, encoding ADA2 protein, one of the adenosine deaminase group enzymes (Lee, 2018; Meyts & Aksentijevich, 2018). Although ADA2 function is yet to be fully elucidated, it has been suggested that ADA2 deficiency results in the increased expression of inflammatory macrophages (reversing the M1/M0 ratio) and has effects on the endothelium. Increased serum levels of ADA2 in infections, autoimmune diseases, and malignancies suggest that it has a function in the regulation of inflammation (Gakis, Calia, Naitana, Pirino, & Serru, 1989; Ratech, Martiniuk, Borer, & Rappaport, 1988; Sari, Taysi, Yilmaz, & Bakan, 2003). Besides, ADA2 gene has been shown to be more expressed in macrophages and monocytes than in other cells. It has also been shown to be effective in macrophage differentiation and cell proliferation by acting as a growth factor (Zavialov et al., 2010; Zhu et al., 2017).
DADA2 was first described in 2014 in a group of patients who were evaluated for recurrent fever, vasculopathy-related skin findings, and ischemic events (Navon Elkan et al., 2014; Zhou et al., 2014). In time with further identification of new patients broad phenotypic variability with autoinflammatory, immunological and hematological findings (Moens et al., 2019) along with a variability in the age of onset were revealed. In some patients, immunodeficiency and hematological findings were evident even before the onset of characteristic vasculopathy-related findings (Schepp et al., 2017).
Vasculopathy, the main cause of morbidity in DADA2, usually involves small and medium sized arteries (Meyts & Aksentijevich, 2018; Moens et al., 2019). Recurrent fever, arthralgia, myalgia, digital necrosis and skin findings including livedoid, macular rash, livedo racemosa, and ulcerations are among the vasculopathy-associated symptoms (Moens et al., 2019). Lesional skin biopsies display vasculopathy-related findings but are far from being diagnostic. However patients may be asymptomatic for years.
The recurrent eczematous lesions in the present patient yet hitherto undescribed in NS-LAH syndrome was probably due to ADA2 mutation. Skin involvement with recurrent pneumonia due to immunodeficiency was the first emerging symptom of DADA2 in our patient.
Acute and chronic lacunar infarcts in association with vasculopathy, presenting with sudden onset ataxia, diplopia, paraplegia, and encephalopathy-related symptoms (Lee, 2018; Moens et al., 2019) may lead to morbidity and mortality in DADA2 (Meyts & Aksentijevich, 2018; Moens et al., 2019). Despite coagulopathy-related problems have not been identified in these patients, the incidence of intracranial hemorrhage has been reported to be increased (Zhou et al., 2014). Central and peripheral neuropathy, cranial nerve paralysis, and hearing loss are among the other reported neurological findings (Batu et al., 2015; Nanthapisal et al., 2016; Poswar Fde et al., 2016). The present patient has not experienced vasculopathy findings so far, however close follow-up was planned in terms of increased risk in neurological complications.
Hematological system involvement, including anemia, thrombocytopenia, leukopenia, and lymphoproliferative disease, constitutes another part of the phenotypic spectrum and patients may present with symptoms with variable severity (Jones, Bazzazi, Kargacin, & Colyer, 2006; Moens et al., 2019). In our patient, hematologic evaluation were all normal but close follow-up was planned. Individuals with biallelic ADA2 pathogenic variants may remain asymptomatic until adulthood or may never develop clinical manifestations of DADA2. While vasculitis usually begins before age 10 years, hematologic disorders may begin early in life or in late adulthood. The timing is as expected in this patient.
Immune system dysfunction has recently been reported more frequently in DADA2 (Schepp et al., 2017; Zhou et al., 2014). It is noteworthy that in half of these patients, symptoms related to immunodeficiency occur prior to symptoms associated with vasculopathy (Schepp et al., 2017). IgM deficiency, panhypoglobulinemia and lymphopenia especially affecting B lymphocytes, are essential laboratory findings in DADA2 related immune deficiency (Lee, 2018; Moens et al., 2019). Immunodeficiency related recurrent herpes simplex virus and sinopulmonary infections have been reported to contribute to morbidity and mortality as was the case in our patient. In the present patient hypoglobulinemia and lymphopenia were attributed to DADA2.
Anti-TNF agents and stem cell transplantation are the current treatment approaches in DADA2. Anti-TNF agents such as Etanercept, Adalimumab, and Infliximab have effects in reducing the frequency of stroke attacks and providing improvement in B cell number and functions (Moens et al., 2019; Schepp et al., 2017). However, these treatments may still remain insufficient in patients with severe cytopenia and immunodeficiency (Hashem et al., 2017; Rama et al., 2018) in whom stem cell transplantation may be an option in the presence of a suitable donor (Hashem, Kelly, Ganson, & Hershfield, 2017; Moens et al., 2019; Zhou et al., 2014).
So far, about 61 mutations in ADA2 have been reported in association with DADA2 (Moens et al., 2019). Missense, nonsense and splice-site mutations are the leading groups. A genotype–phenotype correlation in DADA2 has not been established so far. The novel missense p.Pro193Leu variant detected in our patient further expands the mutational spectrum of DADA2.
In countries with a high consanguineous marriage rate like Turkey (23.1%; Hacettepe University Institute of Population Studies 2014), consanguinity not only increases the risk of rare autosomal recessive disorders but also poses a real challenge in identifying patients with complex phenotypes particularly in the presence of multiple pathogenic variants. Recent studies have identified rare coding variations in consanguineous families, that may never occur without inbreeding (Fujikura, 2016; Kiezun et al., 2012; Ku, Naidoo, & Pawitan, 2011). In addition, multiple diagnosis rates in the same individual are reported as high as 4.9% in whole exome studies in cohorts with parental consanguinity (Lal et al., 2016; Posey et al., 2017).
In conclusion, we described a NS/LAH patient with ADA2 deficiency. While the dysmorphic findings led to the diagnosis of NS-LAH, a detailed evaluation of additional findings suggested the diagnosis of DADA2, which was the main cause of morbidity. The molecular diagnosis of DADA2 has drastic effects in terms of accurate treatment and follow-up in our patient. The co-occurrence of rare entities should be kept in mind in patients presenting with unusual findings for a preliminary diagnosis, especially in the presence of parental consanguinity.
ACKNOWLEDGMENT
The authors would like to thank our patient and his family.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




