Homozygous variants in MAPRE2 and CDON in individual with skin folds, growth delay, retinal coloboma, and pyloric stenosis
Abstract
Cases with multiple molecular diagnoses are challenging to diagnose clinically, yet may be resolved by unbiased exome sequencing analysis. We report an infant with developmental delay, severe growth delay, dysmorphic features, and multiple congenital anomalies including retinal coloboma, congenital pyloric stenosis, and circumferential skin creases. Exome sequencing identified a homozygous missense variant in MAPRE2 and a homozygous stopgain (nonsense) variant in CDON. Variants in MAPRE2, encoding a regulator of microtubule dynamics, lead to congenital symmetric circumferential skin creases type 2, with associated dysmorphism, small growth parameters, and congenital cardiac and genital anomalies. Monoallelic variants in CDON, encoding a coreceptor for sonic hedgehog, have been associated with autosomal dominant pituitary stalk interruption syndrome and holoprosencephaly. Cdon−/− mice have multiple eye defects including coloboma, consistent with the observed human phenotype. Thus, the complex phenotypic presentation of the infant may potentially be attributed to a dual molecular diagnosis. Furthermore, we present CDON as a candidate gene for coloboma formation in addition to the known holoprosencephaly phenotype, and propose to expand the allelic spectrum of CDON to variants associated with autosomal recessive inheritance in addition to dominant inheritance.
1 INTRODUCTION
Congenital symmetric circumferential skin creases [MIM 616734] is a rare disorder characterized by ringed creases of excess skin, intellectual disability, and recognizable facial dysmorphism. Cleft palate, cardiac and genital anomalies are frequently encountered (Isrie et al., 2015; Wouters et al., 2011). Pathogenic variants in either MAPRE2, encoding a member of the microtubule end-binding family of proteins, or TUBB, encoding a β-tubulin isotype, have been shown to underlie this syndrome, apparently through perturbation of microtubule dynamics. Both biallelic and monoallelic variants have been identified in MAPRE2, and functional studies in zebrafish suggested that the variants most likely perturb patterning of the branchial arches through excessive activity (in the recessive cases) or through haploinsufficiency (dominant de novo cases) (Isrie et al., 2015).
CDON encodes a cell surface receptor that functions as a coreceptor for sonic hedgehog (SHH) and mediates cell–cell interactions. In humans, heterozygous CDON pathogenic variants lead to pituitary stalk interruption syndrome and holoprosencephaly (HPE) or holoprosencephaly Type 11 [MIM 614226], inherited in an autosomal dominant manner with variable expression or partial penetrance. Functional studies suggest that the pathogenic mechanism is associated with a loss of ability to promote SHH signaling (Bae et al., 2011). Multiple other HPE genes and their encoded proteins also converge along the SHH pathway, including SHH, PTCH1, and GLI2.
Genome-wide analyses such as whole exome sequencing (WES) allow for hypothesis-free interrogation of phenotypes, leading to increased discoveries of multilocus variation. Blended phenotypes result from pathogenic variation in two or more genes, and provide a molecular alternative to the previously termed phenotypic expansion (Karaca et al., 2018; Posey et al., 2017). We describe an infant with a clinical diagnosis of congenital symmetric circumferential skin creases. However, the affected individual also had congenital pyloric stenosis documented already at 4 days of life, retinal and optic colobomas, and very severe growth failure. WES identified a homozygous missense variant in MAPRE2, compatible with the circumferential skin creases, and an unlinked homozygous stopgain variant in CDON. We suggest that CDON biallelic variants may lead to phenotypic features distinct from those associated with CDON monoallelic variants.
2 MATERIALS AND METHODS
2.1 Exome analysis
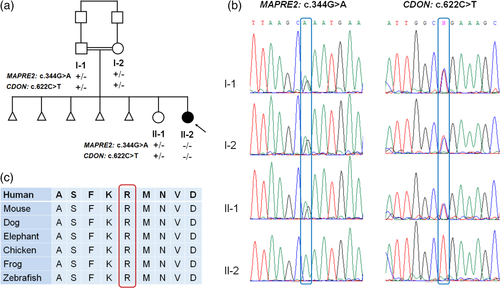
Informed consent was obtained from the family, in accordance with institutional review board (IRB) guidelines. Exonic sequences from DNA of the proband (Figure 1a) were enriched with the SureSelect Human All Exon 50 Mb V5 Kit (Agilent Technologies, Santa Clara, CA). Sequences were generated on a HiSeq2500 (Illumina, San Diego, CA) as 125-bp paired-end runs. Read alignment and variant calling were performed with DNAnexus (Palo Alto, CA) using default parameters with the human genome assembly hg19 (GRCh37) as reference. Exome analysis of the proband yielded a mean coverage of 79X.
2.2 Segregation analysis
Amplicons containing the MAPRE2 and CDON variants were amplified by conventional PCR of genomic DNA, and analyzed by Sanger dideoxy nucleotide sequencing.
3 RESULTS
3.1 Clinical report
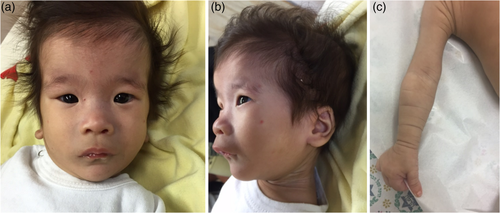
The proband was a 9.5-month old female, the second daughter of a consanguineous couple (first cousins) of Arab Muslim ethnicity (Figure 1a). Parental reproductive history was significant for five early miscarriages. Chromosome analyses for both parents were reported normal. The affected individual was born at 36 3/7 weeks of pregnancy by elective Caesarean section due to intrauterine growth retardation (IUGR) and previous C-section. Birthweight was 2,418 g (26th percentile) and head circumference was 33.3 cm (70th percentile). Physical exam revealed hypotonia and dysmorphic features including bitemporal narrowing, tall and flat forehead, sparse eyebrows, narrow palpebral fissure, epicanthal folds, broad nasal bridge, prominent premaxilla, vaulted palate, micrognathia, and low-set posteriorly rotated ears (Figure 2). Redundant, circumferential skin folds were noted on all limbs, and she had bilateral 5th finger clinodactyly and abnormal female genitalia.
A complicated gastro-intestinal course started at age 4 days, when she presented with vomiting and respiratory distress and was diagnosed with severe hypertrophic pyloric stenosis (Figure S1 ). She underwent surgical repair at 10 days of life. Following surgery, vomiting resolved yet she had bloody stools, and was suspected to have milk-protein allergy. An elemental diet led to improvement. At 4 months, she weighed 3.4 kg (Z-score −4.04) and her length was 52.6 cm (Z-score −3.59). Dietary evaluation suggested severe failure to thrive (FTT) despite appropriate caloric intake for weight and length. Alpha-1-antitrypsin and elastase levels in the stool were normal, as were transferrin electrophoresis and basic metabolic screening tests. Gastroscopy and colonoscopy with biopsy showed villi flattening in the duodenum and a small amount of apoptotic bodies in the colon. A week-long trial of glucocorticosteroids was initiated on suspicion of autoimmune enteropathy, to no avail. Most recent growth parameters at 9.5 months showed weight of 3.9 kg (Z-score −7.09), length 57.5 cm (Z-score −4.49), and head circumference 39.2 cm (Z-score −4.25).
Developmentally, at 9.5 months the infant smiled, laughed, babbled, and supported her bottle for feeds. She could roll from stomach to back from age 8 months, and could scoot on her back. Formal neurocognitive evaluation was not available. Brain ultrasound after birth and head MRI at 8 months of age were normal, including the sella structures and the pituitary gland.
Additional evaluations revealed a small muscular ventricular septal defect, bilateral optic nerve coloboma and unilateral retinal coloboma. Renal ultrasound was normal. The infant's complicated presentation, including dysmorphic features, severe FTT and multiple congenital anomalies, in addition to the recurrent pregnancy losses, prompted genetic evaluation. Chromosomal microarray was normal, and exome sequencing was pursued.
3.2 Exome sequencing identifies homozygous variants in MAPRE2 and CDON
Upon exome sequencing of the proband two variants of potential clinical significance were identified (Figure 1b). The first, a homozygous variant in MAPRE2 (chr18:g.32677539G>A [hg19]; NM_001143827.2: c.344G>A; p.Arg115Gln), is predicted to alter a highly conserved residue (GERP 5.88) (Figure 1c) and is predicted pathogenic by multiple bioinformatics algorithms. The identified MAPRE2 variant was absent from the GnomAD database and from our in-house exome database comprised of ~3,500 individuals with approximately 50% Arab Muslims.
The second variant, a homozygous stopgain variant in CDON (chr11:g.125888243G>A [hg19]; NM_001243597.1: c.622C>T, p.Arg208Ter), is predicted to result in a premature termination codon in exon 5 of 20 exons. The CDON variant was seen in the heterozygous state in three individuals in the GnomAD database and in two unrelated individuals in our internal database of ~3,500 individuals. The probability of loss of function intolerance of CDON is 0.00, indicating that haploinsufficiency is not a likely pathogenetic mechanism. This is consistent with the lack of phenotype in the parents who are carriers of the stopgain variant. Segregation studies revealed that the father, mother, and unaffected sister were heterozygous carriers of both the MAPRE2 and CDON variants (Figure 1b). Thus, we expand the CDON allelic spectrum to include biallelic variants.
4 DISCUSSION
In this study, we report an individual with dysmorphic features, multiple congenital anomalies and severe postnatal progressive growth delay, in whom genetic analysis revealed homozygous likely pathogenic variants in MAPRE2 and CDON, suggestive of a dual molecular diagnosis. The incidence of multiple molecular diagnoses has been reported as 3.2–7.1% of individuals provided a molecular diagnosis (Posey et al., 2017), possibly an underestimate resulting from incomplete molecular analysis after identification of the first molecular diagnosis. In this individual, only the combined features of MAPRE2 and CDON could explain the composite phenotypic presentation. While pathogenic variation in MAPRE2 has been reported with both autosomal dominant as well as recessive inheritance (Isrie et al., 2015), variants in CDON have hitherto been associated with dominant inheritance alone (Bae et al., 2011). Notably, MAPRE2 and CDON reside on two separate chromosomes, such that the genes are not in linkage disequilibrium.
MAPRE2, localized on chromosome 18, encodes a member of the microtubule end-binding family of proteins that bind to the guanosine triphosphate cap at growing microtubule plus ends and either contribute to the regulation of microtubule dynamics or to microtubule reorganization during cell differentiation (Goldspink et al., 2013; Isrie et al., 2015). Monoallelic and biallelic variants in MAPRE2 lead to congenital symmetric circumferential skin creases type 2 [MIM 616734], a syndrome presenting with recognizable dysmorphism and congenital symmetric circumferential skin creases, inherited in either an autosomal dominant or recessive fashion. Other variable features of the syndrome include microcephaly, short stature, varying levels of developmental delay, and cardiac and genital anomalies. Similar to the previously reported biallelic variants, the identified variant herein also resides within the calponin-homology domain, important for interaction with microtubules (Isrie et al., 2015). The role of MAPRE2 in intestinal epithelial differentiation (Goldspink et al., 2013) is of interest, given the villous atrophy demonstrated in this case. Notably, the MAPRE2 variant could explain many, but not all, of the infant's features.
The second potentially causative homozygous variant was identified in CDON. The encoded protein contains three fibronectin Type III domains and five immunoglobulin-like C2-type domains (Kang et al., 1997). It is highly expressed in the frontonasal and maxillary processes of the developing mouse embryo, structures that contain signaling centers that pattern the face (Cole & Krauss, 2003). In rodent models, it is critical for early forebrain and eye development, facial patterning, and myogenesis (Kang, Mulieri, Miller, Sassoon, & Krauss, 1998; Zhang et al., 2009; Zhang, Kang, Cole, Yi, & Krauss, 2006). Although ocular anomalies have not been described in humans with CDON variants, Cdon−/− mice display multiple eye defects including coloboma and microphthalmia (Zhang et al., 2009), consistent with the ocular phenotype of the reported affected individual.
Infantile hypertrophic pyloric stenosis is a complex disorder that results from genetic and environmental factors. Possible pathways in syndromic pyloric stenosis are varied, and include neuromuscular disorders, connective tissue disorders, metabolic issues, ciliary malfunctioning, DNA repair disturbances, MAPK-pathway disturbances, lymphatic abnormalities, and others (Peeters, Benninga, & Hennekam, 2012). Among the molecular pathways involved, BMP4 signaling from the small intestine is required to induce expression of Nkx2.5, a homeodomain gene, in the adjacent gizzard mesoderm; Nkx2.5 in turn is necessary for pyloric sphincter formation. Along with its role in sphincter development, BMP4 controls muscle wall thickness in the gastrointestinal tract by decreasing cell proliferation and increasing apoptosis in the mesoderm layer (Schilling, Concordet, & Ingham, 1999). Additionally, Shh null mice and humans with hypertrophic pyloric stenosis (HPS) exhibit antral epithelial overgrowth (Kolterud et al., 2009). Thus, one might hypothesize that SHH-signaling pathway mutations might promote proliferation in the adjacent mesoderm and may cause pyloric stenosis, although this requires functional studies to refute or substantiate. Alternatively, the pyloric stenosis may be unrelated to the mentioned variants and may reflect as yet unidentified genetic or environmental factors.
In conclusion, we present an infant with a complex phenotypic presentation potentially attributed to a dual molecular diagnosis in CDON and MAPRE2. We suggest that homozygous loss-of-function variants in CDON lead to a phenotype including ocular malformations. Due to obscuring of the phenotype by the second molecular diagnosis, this case cannot reliably define the specific features associated with biallelic variants in CDON. Further studies in humans will likely shed light on the full phenotypic and allelic spectrum of CDON-associated variants.
ACKNOWLEDGMENTS
The authors wish to thank the family for their participation in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Informed consent was obtained from the family, in accordance with institutional review board (IRB) guidelines. Written consent was provided for publication of photographs.
Open Research
DATA AVAILABILITY STATEMENT
The ClinVar accession numbers for the DNA variant data reported in this manuscript are SCV000920379 and SCV000928313.




