Genetic diagnosis of Down syndrome in an underserved community
Abstract
It is a matter of course that in high-income countries, infants born with features suggestive of Down syndrome (DS) are offered genetic testing for confirmation of a clinical diagnosis. Benefits of a definitive diagnosis include an end to the diagnostic odyssey, informed prognosis, opportunities for caregiver support, inclusion to social support networks, and more meaningful genetic counseling. The healthcare experience for families of children born with DS in low- and middle-income nations is in stark contrast with such a level of care. Barriers to obtaining genetic diagnosis might include economic disparities, geographical isolation, and lack of access to health care professionals trained in genetic medicine. As part of a combined research and community outreach effort, we provided genetic testing for several patients with DS. These individuals and their families live on several resource-limited Caribbean islands and have either limited or virtually no access to medical genetics services. Within this group were three families with recurrent DS. Karyotype established that translocation events were not involved in the DS in any of these families. This information enabled genetic counseling to help family members understand their recurrent DS. A definitive diagnosis of DS is beneficial to families in resource-limited communities and may help to provide such families with genetic counseling, reassurance, and peace of mind.
1 INTRODUCTION
Informing and confirming a diagnosis of Down syndrome (DS), either prenatally or at birth, is a common event taken for granted in high-income countries. It is a fundamental part of the fabric of clinical genetics, and medical students learn of its importance early in training. Physicians, genetic counselors, and parents all benefit from an early diagnosis. People who live in low- or middle-income countries have difficulty accessing medical genetics services. The challenges imposed by barriers to diagnostic confirmation or referral to a geneticist or genetic counselor are daunting and affect both families and the healthcare system. These challenges are examples of how socioeconomic status can affect the lives of patients and their families. We are trying to address this disparity in underserved island communities in the West Indies.
Low-income communities comprised of people who are not of Caucasian descent face additional challenges. Most books and journals publish clinical photos and phenotypic analyses of patients who are of European ancestry, but these resources are lacking for peoples of other ethnic origins. Because of this disparity, the diagnostic challenges facing the physician caring for children who have congenital malformations in resource-poor countries include the phenotypic recognition (clinical diagnosis) as well as the underlying genetic cause of the condition. To address this, a project to create an electronic atlas of human malformation syndromes in diverse populations was initiated (Muenke, Adeyemo, & Kruszka, 2016).
We recently participated in an international project to help describe the variation in Down syndrome (DS) phenotypes in diverse populations. The ongoing aim of this project is to create a compendium of human malformation conditions in peoples of diverse ancestry (https://www.nih.gov/news-events/news-releases/nih-creates-atlas-human-malformation-syndromes-diverse-populationshttps://www.nih.gov/news-events/news-releases/nih-creates-atlas-human-malformation-syndromes-diverse-populations). Our contribution to this collaborative effort was to identify individuals with DS from the Caribbean region. For this project, we obtained genetic confirmation of clinically diagnosed DS in 11 patients (Kruszka et al., 2017) from a research testing laboratory. Because of budgetary constraints, we used cytogenomic microarray analysis (CMA) to test for copy number variation (CNV) from blood samples shipped via FedEx to a collaborating laboratory. Testing by CMA can confirm DS but will not distinguish trisomy 21 caused by nondisjunction from DS caused by a Robertsonian translocation. A standard karyotype would have yielded this information, but we were unable to offer it due to lack of funding.
Within our cohort of 11 patients were three separate cases of recurrent DS in different families. Since we diagnosed all patients by CMA, we were unable to offer advice about possible recurrence risk to other family members due to possible Robertsonian translocation. We wanted to pursue karyotype analysis to help the other individuals in these three families understand their recurrent Down syndrome. To this end, we established a second collaborative partnership to secure standard karyotypes.
The purpose of this article is to share our experiences regarding the difficulty of offering genetic testing and genetic counseling in these underserved communities. In addition, we describe how we further characterized the DS in these three families.
2 METHODS
Consent was obtained from parents of all patients, and the individuals tested for DS provided assent. We used a consenting form that was constructed under the auspices of our local institutional review board (IRB) to best relate to the local customs of our patients and families. This is a Caribbean community IRB that is registered with the Office of Human Research Protections (OHRP) in the U.S. Department of Health and Human Services, (IRB Registration Number: IRB00010095). All authors received USA National Institutes of Health (NIH) approved training for human subjects research. Additionally, we used an NIH-created consenting form specific for the atlas of diverse populations project (https://research.nhgri.nih.gov/atlas/consent.shtmlhttps://research.nhgri.nih.gov/atlas/consent.shtml). During the consenting process, we also took family histories from unaffected family members to create pedigrees.
Blood was drawn from select parents and patients into sodium heparin vacutainer tubes. Samples were sent in an insulated box at ambient temperature by FedEx to Baylor Genetics Laboratories (Houston, TX) for karyotype analysis using standard protocols. Analysis was done on a minimum of 20 metaphase cells. In the two cases where we thought that mosaicism might be a possibility, we extended the analysis to 50 metaphase cells plus 100 interphase cells with a chromosome-21-specific FISH probe. Test results were communicated to families under the auspices of local licensed physicians.
3 RESULTS
Standard karyotype analysis showed no Robertsonian translocation in any of the three families (Figure 1). In Family 1, the first child with DS was born when the mother was 25-years-old, and the fourth child, also with DS, was born when the mother was 37. Because there were only four children in this sibship, and 2 had DS, we asked if the mother (I-2) might have a low level of mosaicism. To answer this, we extended our analysis to a total of 50 metaphase cells, and 100 interphase cells with a chromosome-21-specific FISH probe. All cells analyzed were normal, indicating that in Family 1, I-2 does not have detectable peripheral blood cell mosaicism for trisomy 21. For Family 1, we attributed the recurrence to a chance event that was made more likely by the advanced age of the mother when she had her second DS child. Of course, we cannot rule out the possibility of germline mosaicism for I-2. Alternatively, she might have some other genetic predisposition for chromosomal nondisjunction.
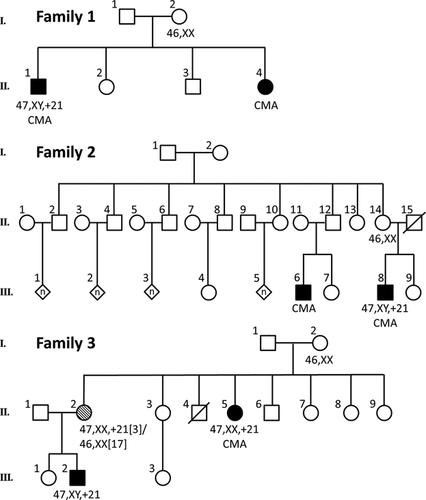
The pedigree of Family 2 depicts first cousins with DS. Individual III-8 was born to a 27-year-old mother, and his cousin with DS (III-6) was born to a mother who was 36 years of age. The karyotypes for this extended family suggest that the second case of DS occurred by chance, which was made more likely by advanced maternal age. Because of the large number of individuals in this family, and because one of the DS patients was born to a mother of advanced maternal age, we did not do any additional analysis to look for mosaic karyotypes.
In Family 3, II-5 was born with DS from a 27-year-old mother. The sister of this patient (II-2) gave birth to a child with DS (III-2) when she was 19-years-old. Karyotype showed that the DS in these two patients (an aunt and her nephew) was caused by trisomy 21, demonstrating that a chromosomal translocation event was not involved in the recurrence of the disorder in this family. Since both mothers conceived a DS child when they were relatively young, we could not explain the recurrent DS by increased risk from advanced maternal age.
Because I-2 had both a child and a grandchild with DS, we looked at her karyotype more closely. In I-2, we extended the metaphase analysis to 50 cells and looked at an additional 100 interphase cells with a chromosome-21-specific probe. All cells analyzed were normal. From this we conclude that, at least in the peripheral blood cells, I-2 does not have a mosaic DS karyotype.
We also obtained karyotype for II-2 in Family 3 that showed a 47, XX, +21[3]/46, XX[17] karyotype. This suggests that the second case of DS in this extended family was made more probable because of the isolated mosaicism for trisomy 21 in II-2. Individual II-2 does not have any observable phenotypic features suggestive of DS. The mosaic karyotype seen in II-2 might have been caused by a post-zygotic mitotic event, or it might have been caused by trisomy rescue of a de novo DS conception (Papavassiliou, Charalsawadi, Rafferty, & Jackson-Cook, 2015).
These findings allowed us to counsel II-2 that recurrence risk for DS to one of her future children would likely be a combination of her maternal age and her mosaicism. Since we cannot ascertain if she has germline mosaicism, we are unable to give a precise recurrence risk estimation for this person.
Our findings allowed us to inform the other members of the three families shown in Figure 1 that their recurrence risk for DS is likely to be the age-adjusted population norm. The knowledge that the many siblings and cousins in these families did not have to be concerned about being carriers of a chromosomal translocation leading to a high recurrence risk for DS was gratefully received by all family members.
4 DISCUSSION
In total, our efforts have so far allowed us to provide a genetic diagnosis of DS for 12 patients who previously had severely restricted access to this testing. This effort was initiated as part of an international collaboration (Kruszka et al., 2017), where we used CMA to provide genetic confirmation to parents of 11 clinically diagnosed DS children. This piece describes how we used karyotype to obtain an additional DS diagnosis and to show that Robertsonian translocation was not involved in the recurrent DS seen in three different families. We conclude that the DS recurrence in two of the families occurred by chance, which was increased by advanced maternal age. In a third family, we found that an isolated case of mosaicism played a role in the recurrence of a second DS child. Importantly, ruling out translocation improved the clinical advice and recurrence risk estimates given to the extended members of these 3 families.
The various island regions where we are tracking DS have a total population of approximately 100,000. We know of more than 50 other suspected DS patients in these communities. A diagnosis of DS is not automatic for every child who has dysmorphic features, mental impairment, or some other congenital anomaly in this community. However, it would be interesting to know how often our clinical diagnosis of DS is confirmed by a genetic test. The next step in this project will be to obtain karyotype for our 50 suspected DS cases. By offering karyotype to these patients we would be able to estimate the rate that our clinical diagnosis of DS is incorrect. A side benefit would be that the parents of our DS patients would have access to genetic diagnostic services.
We maintain continued community involvement with families who are raising children with DS to offer support, management, monitoring, and medical services. The parents who were involved in this project unanimously appreciated that we obtained a genetic diagnosis for their children. Here, we provide a seminal example of why the importance of establishing a medical diagnosis is axiomatic in the field of medical genetics.
The diagnosis we provided to the parents of the patients with DS was welcome news. For these patients, a genetic diagnosis is difficult or impossible to obtain due to geographic isolation, lack of genetic diagnostic services, and economic disparities. These patients and their families reside on small Caribbean islands that have relatively low gross domestic products and small populations. At the request of our IRB, the precise location of these patients will not be revealed to protect the privacy of the patients and their families. Although high quality primary care and a variety of specialty services are offered, access to genetic services is typically unavailable. To access genetic services and/or genetic testing, most patients would have to fly off-island to Canada, Europe, or the United States to consult with a clinical geneticist or a genetic counselor. Each parent of our patients with DS was grateful to know that they no longer had to wonder if their child had another disorder. One mother simply stated, “It's nice to know what I'm dealing with and that I don't have to wonder if it's something else.” This is a pan-ethnic example of the value of accurate diagnosis and illustrates the need for genetic services in communities where they are not available
ACKNOWLEDGMENT
We thank the patients and their families who participated in this project.
ETHICS OVERSIGHT
This project was conducted under the auspices of the St. George's University Institutional Review Board and approved as IRB proposal 13061. This is a Caribbean community IRB that is registered with the Office of Human Research Protections (OHRP) in the U.S. Department of Health and Human Services, (IRB Registration Number: IRB00010095). All authors received USA NIH approved training for human subjects research.