Male child with somatic mosaic Osteopathia Striata with Cranial Sclerosis caused by a novel pathogenic AMER1 frameshift mutation
Abstract
Osteopathia striata with cranial sclerosis (OSCS; OMIM #300373) is a rare X-linked dominant condition caused by mutations in the AMER1 gene (also known as WTX or FAM123B). It is a condition which usually affects females in whom the clinical phenotype can be extremely variable. Conversely affected males typically die in utero or during the neonatal period [Perdu et al. (2011); Clinical Genetics 80: 383-388; Vasiljevic et al. (2015); Prenatal Diagnosis 35: 302-304]. There have been a small number of reported cases of surviving males, including three patients who are somatic mosaic for the condition [Chénier, Noor, Dupuis, Stavropoulos, & Mendoza-Londono, (2012); American Journal of Medical Genetics Part A 158A: 2946-2952; Holman et al. (2011); American Journal of Medical Genetics Part A 155A: 2397-2408; Joseph, Shoji, & Econs, (2010); The Journal of Clinical Endocrinology and Metabolism 95: 1506-1507]. We report a case of a male child who has proven somatic mosaicism for OSCS associated with a novel pathogenic frameshift mutation, c.607_611delAGGCC (p.Arg203 fs) in AMER1. We describe the multisystemic clinical features which include macrocephaly with ventriculomegaly and requirement for ventriculoperitoneal shunt, cleft palate, and respiratory difficulties after birth requiring tracheostomy insertion, persistent patent ductus arteriosus, failure to thrive and gastrostomy insertion, growth retardation, ophthalmoplegia, kidney malformation, cryptorchidism, and developmental delay. The use of new technologies with next generation sequencing (NGS) may improve the detection rate of mosaicism in rare conditions.
1 INTRODUCTION
Osteopathia striata with cranial sclerosis (OSCS; OMIM #300373) is a rare X-linked dominant condition which is caused by mutations in the AMER1 gene (APC membrane recruitment protein 1), also called WTX (Wilms tumor on the X chromosome), or FAM123B (Jenkins et al., 2009). The AMER1 protein acts as a negative regulator of canonical WNT signaling by promoting the ubiquitination and degradation of b-catenin (Major et al., 2007). AMER1 was shown to be a contributor to the skeletal ossification defects observed in mice and humans when WNT signaling is increased (Jenkins et al., 2009). In addition, recurrent somatic mutations in AMER1 have been found in Wilms tumors and colorectal cancer, suggesting a role of the AMER1 protein in tumorogenesis (Rivera et al., 2007; Sanz-Pamplona et al., 2015).
OSCS is a rare bone dysplasia, which is associated with cranial sclerosis and linear striations in long bones as well as multisystemic clinical features. It is a condition which usually affects females in whom the clinical phenotype can be extremely variable. Conversely affected males typically die in utero or during the neonatal period (Perdu et al., 2011; Vasiljevic et al., 2015). More than 100 cases of OSCS have been reported worldwide but this includes only three male individuals who have been identified as somatic mosaic for the condition (Chénier, Noor, Dupuis, Stavropoulos, & Mendoza-Londono, 2012; Holman et al., 2011; Joseph, Shoji, & Econs, 2010) and one male who has Klinefelter syndrome (Fradin, Collet, Ract, Odent, & Guggenbuhl, 2016). We present the fourth reported case of a male child who is a somatic mosaic for OSCS and describe his phenotype and clinical management.
2 CLINICAL REPORT
A male baby with complex health problems was referred to our clinical genetics team and was first reviewed by us at 17 weeks old. He was born to unrelated healthy parents and had a healthy older brother. There was no relevant family history.
2.1 Pregnancy history
The pregnancy was initially unremarkable with a normal nuchal scan at 13 weeks gestation. A detailed 20 week scan detected borderline ventriculomegaly which was confirmed by an MRI scan at 23 weeks gestation. Polyhydramnios was detected at 26 weeks and amnio-reduction was performed at 28 weeks gestation. The polyhydramnios recurred but further reduction was not recommended. The fetal growth parameters were known to be significantly increased, particularly the abdominal and head circumferences.
The male baby was born by elective Cesarean section at 40 + 3 weeks gestation with a birth weight of 4.62 kg (near 98th centile) and a head circumference of 40.6 cm (nearly 2 cm above the 99.6th centile; +4.24 SD). He was noted to have Pierre-Robin sequence with a large cleft palate and cryptorchidism. He was born with two natal teeth (middle lower incisors). He required immediate intubation after delivery as he was unable to self-maintain his airway. On day 3 of life, a tracheostomy was inserted.
Our patient had three hospital admissions for chest infections in the 1st months of life. A gastrostomy was inserted at 3½ weeks of age and he also underwent a right inguinal hernia repair with a left prophylactic orchidopexy. He had moderate to severe gastrointestinal reflux disease and underwent a Nissen fundoplication. He passed his newborn hearing screen and had a normal initial ophthalmology assessment. There was no history of seizures. A renal ultrasound scan showed a unilateral duplicated renal pelvis. A cranial ultrasound, performed at 4 months of age, showed persistent dilated lateral ventricles but no sign of acute hydrocephalus.
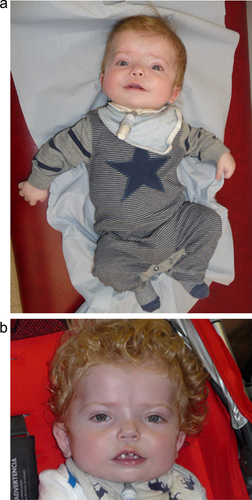
He was reviewed by our genetics team when he was 17 weeks (Figure 1a). He now had a head circumference of 48.5 cm (4 cm greater than 99.6th centile; +4.77 SD) and a weight of 6.92 kg (50th–75th centile). His anterior fontanelle was particularly large measuring 9 × 7 cm, but was normotensive. The baby had significant brachycephaly with frontal bossing, ocular hypertelorism, and a broad nasal bridge. He had a smooth philtrum with low-set ears and micrognathia. He had normal tone but still had some head lag. He had deep palmar creases.
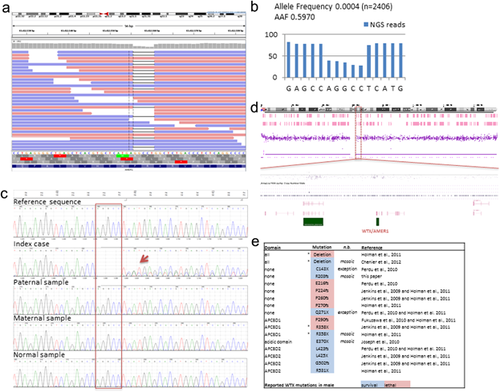
A genetic diagnosis had been suspected and the baby initially had an array-CGH which was normal. Osteopathia striata with cranial sclerosis was considered the most likely diagnosis based on the constellation of multiple congenital anomalies and cranio-facial dysmorphic features. Targeted analysis of AMER1 was therefore requested. DNA extracted from blood was sequenced on the local diagnostic clinical exome pipeline and analyzed for the coding sequence and splice site junctions of the AMER1 gene. The genetic testing revealed a novel pathogenic frameshift mutation, c.607_611delAGGCC in mosaic form of around 60% as shown by the allele proportion of Next Generation Sequencing (NGS) reads (AAF for assay allele frequency, Figure 2a,b). The presence of the mutation and its mosaicism was confirmed by Sanger sequencing (Figure 2c). Examination of the array data on chromosome X for this patient did not reveal copy number changes in the AMER1 region that could explain the mosaicism of the mutation (Figure 2d). The c.607_611delAGGCC is predicted to induce a premature stop codon at position p.205 (p.Arg203fs*2), leading to a truncated AMER1 protein or nonsense-mediated decay of the AMER1 mRNA. The presence of this mutation confirmed a diagnosis of osteopathia striata with cranial sclerosis (OSCS). Testing of the mother confirmed that the mutation had arisen de novo in our patient (Figure 2c).
The baby was referred to the neurosurgical team who arranged a ventriculoperitoneal shunt insertion after detection of raised intracranial pressure. At 1 year of age (see Figure 1b) he was able to clap and point, wave, roll, and to sit for periods without support. On examination his head circumference was 53.5 cm (>99.6th centile), his weight was 9.21 kg (25th–50th centile) and his length was 72.2 cm (9th centile). He had developed ridging over the coronal sutures on either side of his anterior fontanelle, which is likely to have resulted from the cranial sclerosis.
At 18 months of age, his weight was 10.4 kg (25th centile) and his head circumference was 57 cm (+6.23 SD). He could sit without support and was almost able to crawl. He was pointing at objects and had developed a pincer grip. He had bilateral astigmatism and was also noted to have worsening bilateral ptosis (right eye more severe than left) with an intermittent left divergent squint.
By 2.5 years of age, the child had the first stage of the cleft palate repair. He had a persisting patent ductus arteriosus and glue ear. He was able to cruise around furniture but was not yet walking without support. His receptive language was improving and he could understand simple phrases.
2.2 Molecular analyses
Next Generation Sequencing (NGS) of the coding region (±5 bp) of the AMER1 gene (reference sequences NM_152424.3) was performed using the Illumina TruSight One sequencing panel. One hundred percent of the target sequence within this panel was sequenced to a depth of 20-fold or more. This identified the c.607_611delAGGCC change in the AMER1 gene. The presence of the sequence change was confirmed in the child by Sanger sequencing and tested in parental DNA samples extracted from blood. The PCR amplification was performed using standard procedures (primer sequences are available on request) and the sequencing products electrophoresed on an ABI3730 genetic analyzer. Sequence analysis was done using MutationSurveyor. Mutations are named using HGVS nomenclature, where nucleotide one is the A of the ATG-translation initiation codon. Copy number analysis on the X chromosome was performed by array analysis with a genome-wide resolution of 200 kb using Affymetrix Chromosome Analysis Suite (ChAS) software (Build 37).
3 DISCUSSION
Our male patient presented as a case of somatic mosaicism of OSCS. On leucocyte analysis, approximately 60% of cells were found to have a small deletion encompassing five base pairs within the AMER1 gene; namely c.607_611delAGGCC (p.Arg203fs*2). This mutation has not been previously reported. The deletion causes a frameshift which is predicted to cause truncation of the AMER1 protein.
There have been three previous clinical reports of male patients who are somatic mosaic for OSCS. Two of these patients had a single base pair alteration resulting in a nonsense mutation (Holman et al., 2011; Joseph et al., 2010; Lazar, Braunstein, & Econs, 1999). The third patient had a 1.5 Mb microdeletion of Xq11.2 which includes the AMER1 gene, as well as four other known RefSeq genes SPIN4, LOC92249, MIR1468, and ARHGEF9 (Chénier et al., 2012). These mutations are all predicted to result in truncated protein. The clinical features in these patients have previously been outlined by Chénier et al. and we include, in tabular form, the features from our patient (see Table S1 in Supplemental Information). There has also been a recent report of OSCS in an adult male with non-mosaic Klinefelter (XXY) syndrome. This patient had clinical and radiological features of which many resembled the phenotype described in female patients (Fradin et al., 2016).
Our patient had significant medical interventions commencing from the neonatal period. He has ventriculomegaly and renal anomalies which are features that have not been previously reported in the other male cases of mosaicism but which have been described in males with full mutations of OSCS (Holman et al., 2011).
There has been consideration over possible genotype-phenotype correlation regarding survival of males with OSCS, and specifically whether mutations at the 5′-end of AMER1 are associated with a more severe phenotype, than those located at the 3′-end (Jenkins et al., 2009). This correlation has since been shown to not always be consistent (Perdu et al., 2010, 2011). Figure 2e summarizes the amino acid positions of all mutations reported in males together with their associated lethality or survival. The lethality rule exceptions are indicated as well as the germline/mosaic status. Interestingly, two of the reported viable mosaic mutations are lethal when present in germline in males (R358X and gene deletion). However, it is unknown whether this is the case with the novel mutation found in our patient.
In conclusion, the clinical features in our patient adds to the phenotypic spectrum of surviving males with OSCS. Our case demonstrates that somatic mosaic males may present with distinctive dysmorphic features and a constellation of congenital anomalies affecting multiple systems. It also establishes that diagnosis may still be determined clinically, and confirmed by targeted single gene sequencing, even in an era of whole exome and genome sequencing. A multidisciplinary medical approach to management from birth may be crucial for survival in patients such as ours.
ACKNOWLEDGMENTS
We thank the patient's family for their support and consent to this publication. We also thank the multidisciplinary teams at CUHNFT, Peterborough City Hospital, Great Ormond Street Hospital, and Nottingham Fetal Care Unit who are involved in the care of this patient and his family.
DISCLOSURE
The authors have indicated they have no financial relationships relevant to this article to disclose.