Defining mandibular morphology in Robin sequence: A matched case-control study
Abstract
Robin Sequence (RS) is classically defined as the triad of micrognathia, glossoptosis, and airway obstruction. While there remains significant debate over diagnostic criteria for severity, there is consensus regarding micrognathia as a defining feature of the condition. The purpose of this study was to compare mandibular morphology among infants and children with RS to infants and children without RS using maxillofacial computed tomography. Our hypothesis was that there are discrete morphologic differences between RS and non-RS mandibles. Our goal was to determine if there are defined and measureable differences in RS mandible shape that can be used in defining the sequence. We identified 20 cases with RS and 20 age- and sex-matched controls without RS. Linear, angular, and composite measurements were obtained for each patient. Cases had shorter mandibular sagittal lengths (−27%, p = 0.001), shorter inferior border arc lengths (−11.5%, p = 0.002), steeper gonial angles (+10.5%, p < 0.001), and narrower symphyseal angles (−11.5%, p < 0.001). Mandibular shape in RS was more rounded/elliptical (p < 0.001) and infants with RS had a significantly smaller submental cross-sectional area (−29.4%, p < 0.001). These shape differences anterior to the gonial angle of the mandible appear to be a defining morphologic feature in RS.
1 INTRODUCTION
Robin Sequence, a congenital condition occurring in approximately 1:8500 live births, is characterized classically as the triad of micrognathia, glossoptosis, and upper airway obstruction (Basart et al., 2015; Breugem et al., 2016; Evans et al., 2011; Evans, Rahbar, Rogers, Mulliken, & Volk, 2006; Hanson & Smith, 1975; Marques, Barbieri, & Bettiol, 1998; Poswillo, 1967; Robin, 1923, 1934, 1994). While this triad is the cornerstone of the condition, the majority of infants with RS will have a secondary palatal cleft, and over 50% of infants with RS have other anomalies or an associated syndrome, most commonly, Stickler (Caouette-Laberge, Bayet, & Larocque, 1994; Cohen, 1999; Marques et al., 2005). While the wide degree of phenotypic variability in RS has led to a lack of uniformity in diagnostic criteria and the management of this uncommon condition remains variable, there is consensus that micrognathia is the primary characteristic of RS (Breugem et al., 2016).
The characteristic micrognathia in RS has been investigated by several groups (Chung et al., 2012; Figueroa, Glupker, Fitz, & BeGole, 1991; Pfaff, Metzler, Kim, & Steinbacher, 2014; Pruzansky & Richmond, 1954; Rogers, Lim, Mulliken, & Padwa, 2009; Roy, Munson, Zhao, Holinger, & Patel, 2009; Salerno et al., 2014; Suri, Ross, & Tompson, 2006, 2010). Pruzansky and Richmond (1954) assessed mandibular size and position using lateral cephalograms. Figueroa, also using two-dimensional cephalometric analyses, identified differences in mandibular morphology and upper airway diameters, as well as changes in related to growth (Figueroa et al., 1991). Rogers et al. (2009) reported on the differential changes in the Robin mandible in patients with and without an associated syndromic diagnosis. With the adoption of multi-detector computed tomography at many craniofacial centers, more recent reports have focused on differences in mandibular volume between RS and normal mandibles, as well as the effects of treatment on bone and airway volumes (Chung et al., 2012; Pfaff et al., 2014; Roy et al., 2009; Salerno et al., 2014). A recent study by our group identified soft tissue and bony CT measurements associated with receipt of a tracheostomy tube (Lee, Evans, Perez, Oron, & Perkins, 2016). All of these reports have verified the clinical observation that the Robin mandible is shorter and smaller than a normal mandible. However the mandibular morphology in RS remains loosely defined, as there are no discrete quantitative measures defining the shape of the infant mandible. Systematic characterization of pre-treatment mandibular shape in RS will allow for identification of subphenotypes that will inevitably guide etiologic inquiries.
The specific aim of the present study was to assess the morphologic features of the mandible in infants and young children with RS using three-dimensional computed tomography and compare these to age- and sex-matched controls. We hypothesized that there are specific, quantifiable morphologic differences that distinguish the mandible in an infant with RS (RS mandible) from the normal mandible (non-RS mandible).
2 MATERIALS AND METHODS
2.1 Study design/sample
This was a case-control study of infants and children treated at the Seattle Children's Hospital over a 12-year period. Over this period, 137 patients met the clinical criteria for Robin sequence, defined as micrognathia, glossoptosis, and airway obstruction. From this pool of patients, we identified a subset of patients who had a craniofacial computed tomographic scan with multi-planar and three-dimensional reformats as part of a treatment protocol for mandibular distraction. We identified 20 patients who met these criteria. Controls were age- and sex-matched infants and children who had a craniofacial computed tomographic scan for an indication other than a craniofacial or facial skeletal anomaly, facial surgery, or airway surgery. Among the controls, the indications for CT scanning were as follows: 11 patients had scans for evaluation of a clinically evident soft tissue mass (e.g., encephalocele, dermoid cyst); five patients had scans for evaluation of facial injuries (no displaced fractures were identified on the scans); three patients had scans for evaluation of head shape (no evidence of craniosynostoses were found); one patient had a scan for evaluation of feeding difficulty. The project was approved by the institutional review board for human studies.
2.2 Study variables
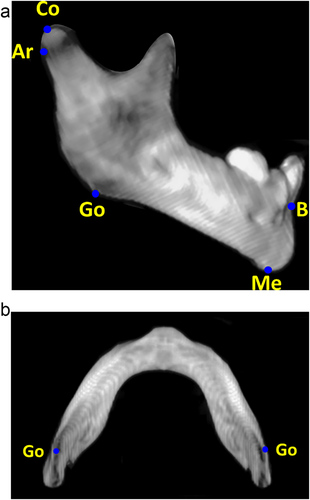
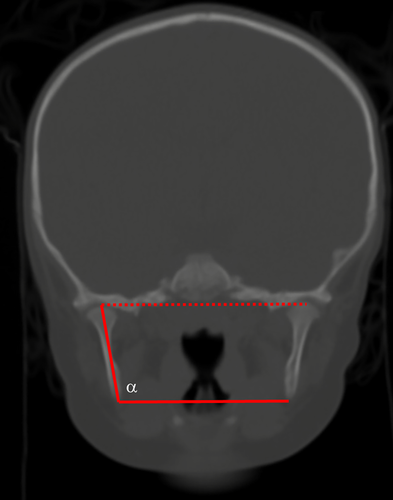
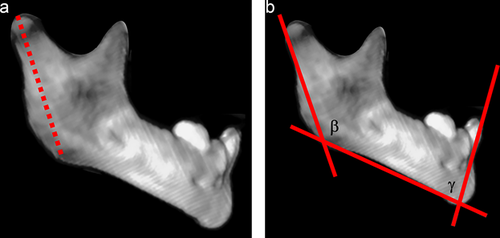
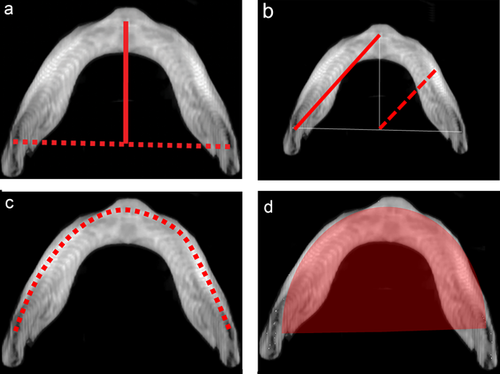
Study measures were quantitative assessments of mandibular morphology, utilizing both accepted anthropometric landmarks and introducing novel, comprehensive shape descriptors. These measures were broadly classified as linear measurements, angular measurements, and composite measurements (Table 1). All patients had multi-detector maxillofacial computed tomography scans (slice thickness 0.625–2.0 mm) with multi-planar (axial, coronal, and sagittal) and three-dimensional reformations. All measurements were performed by the same observer, who was blinded to case-control status. Linear measurements included inter-condylar distance (mm), inter-gonial distance (mm), ramus height (mm), mandibular sagittal length (mm), mandibular radial length (mm), and mandibular inferior border arc length (mm). Angular measurements included ramus angulation (degrees), gonial angle (degrees), and symphyseal angle (degrees). Ramus angulation was measured by using contiguous coronal slices to construct a plane tangent to the most inferior point of the mandibular ramus and a plane passing through the long axis of the ramus. The angle formed by the intersection of these two planes was the ramus angulation. This method was used to accommodate the positioning of the ramus in different coronal slices due to head positioning and/or variability in glenoid fossa anatomy. Composite measurements included mandibular first eccentricity, radial to sagittal length ratio, and submental surface area (mm2). The mandibular first eccentricity was defined as the ratio of the sagittal length to the oblique length, with eccentricities ranging from 0 (circle) to 1 (parabola). Measurements were made using standardized DICOM rendering software (Centricity PACS, ©GE Medical Systems, Little Chalfont, United Kingdom) and three-dimensional cephalometric software (Dolphin Imaging, © Patterson Dental Supply Inc., Redmond, WA). Graphical depictions of the cephalometric points and standardized measurements are shown in Figures 1-4.
| Landmark | Definition |
|---|---|
| Condylion (Co) | Most postero-superior point on mandibular condyle |
| Articulare (Ar) | Junction between the inferior surface of the cranial base and the posterior border of the ascending ramus of the mandible |
| Gonion (Go) | Most postero-inferior point on the angle of the mandible |
| Menton (Me) | Most inferior point on the mandibular symphysis |
| B point (B) | Most concave point on the mandibular symphysis |
| Linear measure | Definition |
| Inter-condylar distance (Co-Co) | Distance between right and left condylion points |
| Inter-gonial distance (Go-Go) | Distance between right and left gonion points |
| Ramus height (Co-Go) | Linear distance between condylion and gonion |
| Mandibular sagittal length | Distance between the midpoint of the mandible at the inferior border to the inter-gonial line |
| Mandibular radial length | Length of a line drawn from the point of intersection of the intergonial line and mid-sagittal plane to the inferior border of the mandible at an angle of 45° relative to both |
| Inferior border arc length | Distance along an arc drawn at the inferior border from gonion to gonion |
| Angular measure | Definition |
| Ramus angulation | Angulation of the long axis of the ramus relative to the inter-gonial plane |
| Gonial angle (Ar-Go-Me) | Angle formed by connecting Ar-Go-Me |
| Symphyseal angle (Go-Me-B) | Angle formed by connecting Go-Me-B |
| Composite measure | Definition |
| First linear eccentricity | Ratio of the mandibular oblique length to the mandibular sagittal length |
| Mandibular radial/sagittal ratio | Composite measure of shape, calculated as the ratio of the mandibular radial length to the mandibular sagittal length |
| Submental surface area | Area bounded by the intergonial plane and an arc drawn along the inferior border of the mandible from gonion to gonion |
2.3 Statistical analyses
Once the measures were validated, de-identified data were iteratively entered into a statistical database (SPSS, v.24.0, © SPSS Inc., Chicago, IL) for analysis. The defined measurements were evaluated for reproducibility as follows: two raters independently evaluated five scans and performed all measurements. Correlation between the two raters was estimated using the intra-class correlation coefficient, with a two-way mixed model testing for absolute agreement. Given the lack of confirmed normality within the dataset, a non-parametric paired samples comparison (Wilcoxon signed ranks test) was utilized to compare measurements between RS mandibles and non-RS mandibles. For all analyses a p-value <0.05 was considered statistically significant.
3 RESULTS
The study sample was comprised of 20 subjects with RS and 20 age- and sex-matched control patients. The mean age was 8.5 ± 9.8 months for controls and 8.2 ± 9.7 months for cases (p = 0.08); there were 13 (65.0%) females in each group. Among the cases with RS, 10 (50%) had isolated RS, and 10 (50%) had an associated syndrome or genetic difference (Stickler n = 4, 20%; auriculo-condylar syndrome n = 2, 10%; major chromosomal abnormality n = 4, 20%). For the measures utilized, there was a high degree of inter-observer agreement (ICC 0.95–0.99, p < 0.001). Intra-class correlation values for each linear and angular measure are listed in Table 2.
| Measurement | ICCa | p |
|---|---|---|
| Inter-condylar distance (Co-Co) | 0.98 | <0.001 |
| Inter-gonial distance (Go-Go) | 0.99 | <0.001 |
| Ramus height | 0.99 | <0.001 |
| Mandibular sagittal length | 0.99 | <0.001 |
| Mandibular radial length | 0.99 | <0.001 |
| Inferior border arc length | 0.98 | <0.001 |
| Ramus angulation | 0.97 | <0.001 |
| Gonial angle (Ar-Go-Me) | 0.98 | <0.001 |
| Symphyseal angle (Go-Me-B) | 0.95 | <0.001 |
- a Intraclass correlation, calculated by comparing measured values from two blinded observers (alpha = 0.05, absolute agreement).
Comparative data for the study population are listed in Table 3. With regard to linear measurements, infants with RS had shorter mandibular sagittal lengths (−27.2%, p = 0.001), shorter mandibular oblique lengths (−13.2%, p = 0.001), and shorter inferior border arc lengths (−11.5%, p = 0.002). The inter-condylar distance, inter-gonial distance, ramus height, and mandibular radial length were statistically equivalent between cases and controls (p > 0.11).
| Controls (n = 20 subjects) | Cases (n2 = 20 subjects) | |||||
|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Δ%a | p-valueb |
| Linear measurements (mm) | ||||||
| Inter-condylar distance | 65.72 | 8.42 | 63.82 | 7.62 | −2.40 | 0.26 |
| Inter-gonial distance | 57.42 | 6.63 | 55.46 | 8.73 | −2.70 | 0.39 |
| Ramus height | 28.34 | 7.72 | 26.95 | 6.65 | −2.93 | 0.14 |
| Mandibular sagittal length | 37.09 | 6.47 | 26.90 | 10.69 | −27.20 | 0.001 |
| Mandibular oblique length | 45.49 | 6.82 | 39.19 | 7.65 | −13.20 | 0.001 |
| Mandibular Radial Length | 73.87 | 7.77 | 69.59 | 22.33 | −5.98 | 0.11 |
| Inferior border arc length | 95.47 | 13.81 | 84.20 | 17.23 | −11.50 | 0.002 |
| Angular measurements (degrees) | ||||||
| Ramus inclination | 104.62 | 4.81 | 100.98 | 4.61 | −3.30 | 0.04 |
| Gonial angle | 131.37 | 4.39 | 145.56 | 7.35 | 10.50 | <0.001 |
| Symphyseal angle | 89.61 | 7.14 | 78.93 | 7.73 | −11.45 | 0.001 |
| Composite measures | ||||||
| First linear eccentricity | 0.81 | 0.04 | 0.67 | 0.16 | −17.40 | 0.001 |
| Radial/sagittal length ratio | 0.75 | 0.07 | 0.97 | 0.10 | 30.30 | <0.001 |
| Submental aurface area (mm2) | 1,695.84 | 454.35 | 1,184.38 | 518.01 | −29.40 | <0.001 |
- a Paired percentage difference expressed relative to cases. Positive percentages suggest that the cases had larger values than the controls; negative percentages suggest that the controls had larger values than the controls.
- b p-values from Wilcoxon signed ranks test (paired analyses with age- and sex-matched controls). Statistically significant (p < 0.05) values indicated in bold
All three angular measurements were significantly different between cases and controls. Subjects with RS had more upright mandibular rami (−3.3%, p = 0.04), steeper gonial angles (+10.5%, p < 0.001), and narrower symphyseal angles (−11.5%, p < 0.001).
Similar differences were observed comparing the composite measurements (p < 0.001). Control mandibles had a mean eccentricity of 0.81, suggestive of a more parabolic shape; RS mandibles had a mean eccentricity of 0.67, consistent with a more elliptical shape (eccentricity difference −17.4%). The rounder mandible in infants with RS was also seen in the radial length/sagittal length ratio. For subjects with RS, the ratio was 0.97, suggestive of a near 1:1 ratio of radial length to sagittal length. For control mandibles, the ratio was 0.75, suggestive of a longer sagittal dimension relative to the radial length. Finally, RS mandibles had a smaller submental surface area (−29.4%, p < 0.001).
4 DISCUSSION
Although Robin sequence has been a well-recognized clinical entity for nearly a century, it remains a complex and poorly understood condition. The interplay between the anatomic distortions and phenotypic variability, as well as the unknown changes in the craniofacial skeleton and airway size over time continue to challenge clinicians. One step toward improving our understanding of RS is to attempt to identify morphologic differences between those with and without the condition. As mandibular hypoplasia is the defining characteristic of the RS phenotype, the purpose of this study was to evaluate pre-treatment mandibular morphology among infants and children with RS (defined as micrognathia, glossoptosis, and airway obstruction) with age- and sex-matched controls using three-dimensional multi-detector computed tomographic scans. We hypothesized that there would be discrete differences in linear, angular, and composite measurements that would distinguish RS mandibular morphology from normal mandibular morphology among infants and children.
The results of this study suggest that there are several discrete differences in mandibular morphology between infants with RS and matched controls. The morphologic differences assessed herein were different in magnitude for measures posterior to the gonial angle versus those anterior to the gonial angle. Posterior to the gonial angle, the magnitude of the differences was relatively small and largely non-significant. RS mandibles had a slightly narrower ramus inclination angle (mean paired difference −3.6 + 6.8 degrees, −3.3%). In contrast, anterior to the gonial angle, more profound differences were identified (Figure 5a–d). The RS mandible was 27% shorter in the sagittal plane (mean paired difference 10.2 ± 9.9 mm), was nearly 20% rounder (mean paired eccentricity difference 0.1 ± 0.2), and had a 30% smaller submental cross-sectional area (mean paired difference 511.5 ± 453.7 mm2).
Primary failure of mandibular outgrowth is a hypothesized model of RS pathogenesis (Tan, Kilpatrick, & Farlie, 2013). The morphometric differences we have described may be related to embryologic events occurring early in mandibular development. Meckel's cartilage, first detected around the 32nd day of gestation, is the first mandibular skeletal component to form (Parada & Chai, 2015; Tan et al., 2013). Ossification begins at approximately 7 weeks, with the initial site of ossification at the division of the inferior alveolar nerve into incisive and mental nerve branches (the site of the mental foramen in the developed mandible, which is located anterior to the gonial angle). Development then continues in both a proximal and distal direction, resulting in formation of the symphysis and ramus-condyle unit via endochondral ossification, with intramembranous ossification in the intervening body of the mandible (Parada & Chai, 2015). The differences observed in this study may be the result of a developmental anomaly at this site of ossification, resulting in constricted growth of the portion of the mandible between the symphysis and ramus-condyle unit. In conjunction with posterior superior displacement of the tongue, this is one explanation for the palate-shaped palatal cleft in RS (Parada et al., 2015; Ricks, Ryder, Bridgewater, Schaalje, & Seegmiller, 2002; Schubert, Jahn, & Berginski, 2005). This hypothesis is consistent with in vivo animal models for the RS phenotype and gene studies demonstrating shared pathways for mandible, tongue, and palate development (e.g., SOX11, ERK/MAPK) (Huang et al., 2016; Parada & Chai, 2015; Parada et al., 2015; Ricks et al., 2002; Schubert et al., 2005). It remains to be seen whether these data can be extrapolated to human mandibular development. As the etiology of the RS phenotype in the majority of our case group is not known, we included cases with isolated RS and with diagnosed Stickler syndrome. As the genetic contributions to mandibular development in humans are realized, it may be appropriate to further characterize these mandibular morphological differences within genotype subgroups. This systematic morphological characterization of the mandible has the potential to facilitate etiological inquiries and genetic discovery for children with the RS phenotype and shared mandibular characteristics.
In addition to offering a potential explanation for the pathophysiology of the RS, data from the current study may explain the observed success of mandibular lengthening for some infants with RS. First, a smaller sagittal dimension with concomitantly lower cross-sectional area could predispose to glossoptosis and tongue-base airway obstruction because of the retro-mandibular soft tissue occupying a smaller space. It is unclear whether the tongue soft tissue dimensions differ between children with and without RS (Figueroa et al., 1991; Hong & Kearns, 2015; Lee et al., 2016). We hypothesize that the mandibular deformity is proportionally greater than the soft tissue deformity, leading to a larger amount of soft tissue relative to the bony skeleton. This would explain the clinical observation that some patients improve with mandibular growth, as well as the growing body of data that demonstrates the effectiveness of mandibular lengthening by distraction osteogenesis (Almajed et al., 2017; Bangiyev, Traboulsi, Abdulhamid, Rozzelle, & Thottam, 2016; Figueroa et al., 1991; Flores et al., 2014; Hong & Kearns, 2015; Papoff et al., 2013; Pfaff et al., 2014; Tahiri, Viezel-Mathieu, Aldekhayel, Lee, & Gilardino, 2014). With regard to distraction osteogenesis, the vast majority of reports demonstrate effective treatment with osteotomies placed at or near the gonial angle. Thus, the mandibular lengthening achieved is at the level of the mandibular body, or anterior to the gonial angle/anti-gonial notch (Almajed et al., 2017; Bangiyev et al., 2016; Figueroa et al., 1991; Flores et al., 2014; Hong & Kearns, 2015; Papoff et al., 2013; Pfaff et al., 2014; Tahiri et al., 2014; Zellner, Mhlaba, Reid, & Steinbacher, 2017). This is consistent with the observations herein, where the most pronounced skeletal differences in the RS case group are anterior to the gonial angle. The effectiveness of distraction may be precisely due to this level of anatomic correction.
There are some limitations to the analyses that merit consideration, so as to frame the results in the appropriate clinical context. First and foremost, the data herein represent only objective measures of the degree of anatomic variation. We did not have systematically collected clinical data for cases and controls to facilitate correlation of clinical findings with the specific degree of anatomic deviation from the norm. As such, we can only provide analyses regarding the differences in morphology; we cannot, at the present time, correlate these differences to the clinical severity beyond stating that the differences observed distinguished subjects with micrognathia, glossoptosis, and airway obstruction from age- and sex-matched controls who had none of these clinical features. Second, the measurements taken in this study represent a single point in time for any given patient. Historically, there has been significant emphasis on the potential “catch up” growth of the mandible as a putative explanation for clinical improvement over time (Bütow, Zwahlen, Morkel, & Naidoo, 2016; Daskalogiannakis, Ross, & Tompson, 2001; Mackay, 2011). Finally, this study represents a small sample of infants with RS (utilizing the consensus definition), who had clinically obtained CT scans in anticipation of possible mandibular distraction. We do use a protocol at our institution that guides decision-making on imaging for infants with tongue based airway obstruction, and patients who are considered for distraction represent those with more significant clinical severity. Therefore, it is very likely that the differences observed herein represent differences between the normal infant mandible and a Robin phenotype that is at the more severe end of a spectrum. As such, the differences seen herein may not be applicable to all children with the RS phenotype. To the extent that mandibular morphology is associated with clinical severity, it is possible that patients with a milder phenotype (i.e., those who improve with positioning alone) may have less notable differences in mandibular morphology relative to normal infants.
Data from this study demonstrate that the RS mandible is shorter in both sagittal length and inferior border arc length, with a flatter symphysis and wider gonial angle. These findings translate into a less parabolic shape with a smaller submental cross-sectional area and may offer insights into the etiology of RS and the mechanism of airway obstruction in this important and understudied condition. These data are the foundation for future studies focused on assessing changes in mandibular morphology that occur as a result of distraction lengthening and using associations between the morphologic and clinical features to predict disease severity in infants and young children with RS.
CONFLICTS OF INTEREST
The authors have no financial or non-financial interests in any of the products, devices, or materials used in this study.