Compound heterozygous mutations in COL1A1 associated with an atypical form of type I osteogenesis imperfecta
Abstract
Heterozygous mutations in the genes encoding the proα1(I) or proα2(I) chains of type I procollagen (COL1A1 and COL1A2, respectively) account for most cases of osteogenesis imperfecta (OI), a disorder characterized by reduced bone strength and increased fracture risk. COL1A1 mutations can also cause rare cases of Ehlers–Danlos syndrome (EDS), a disorder that primarily affects connective tissue and often includes reduced bone mass. Here we present a kindred of three young siblings ages 1–4 years old whose mother has a history of mild type I OI. All three children are compound heterozygotes for COL1A1 mutations, with a novel frameshift mutation (c.2522delC; p.Pro841Leufs*266) from their mother and a known missense mutation (c.3196C>T; p.R1066C) from their clinically unaffected father, which has previously been described as causing a combined type I OI/EDS phenotype. The three children exhibit features of both COL1A1 mutations: early and frequent long bone fractures, joint hyperextensibility, and blue sclerae. We describe three siblings who are the first reported surviving subjects with biallelic pathogenic COL1A1 mutations. They have a more severe form of type I OI with features of EDS that represents their compound heterozygosity for two deleterious COL1A1 mutations. Their long-term outcomes are yet to be determined.
1 INTRODUCTION
Osteogenesis imperfecta (OI) is a connective tissue disorder characterized by reduced bone mass, increased bone fragility, and fractures (Forlino & Marini, 2016; OMIM®, 2016). In the majority of patients with OI (types I–IV), mutations in the genes that produce type I collagen, which is an essential structural component of bone, tendons, skin, sclerae, and dentin, are present. Type I collagen is composed of two α1(I) and one α2(I) chains, which are the mature processed forms of the proα1(I) and proα2(I) proteins encoded by COL1A1 and COL1A2, respectively. A mutation in either of these genes can result in abnormal or insufficient type I collagen and cause defects in bone, tendon, skin, sclera, and dentin composition. Type I OI is typically due to heterozygous null mutations in COL1A1 that cause haploinsufficiency and inadequate proα1(I) production, resulting in frequent fractures during childhood that abate after adolescence (Ben Amor, Roughley, Glorieux, & Rauch, 2013; Forlino & Marini, 2016). In contrast, types II–IV OI are typically due to heterozygous mutations in COL1A1 or COL1A2 that alter collagen structure.
Mutations in other genes involved in the post-translational modification of type I collagen have recently been identified as rare causes of OI. Types V–XVIII OI have been named according to the order in which causative genes have been identified (Kang, Aryal, & Marini, 2017). The growing number of molecular causes of OI is challenging the traditional categorization of OI, which was based on clinical presentation, with type I OI being the least severe, type II OI causing perinatal lethality, type III OI causing severe progressive deformities, and type IV OI causing variable moderate deformities (Sillence, Senn, & Danks, 1979). Therefore, reorganization of the naming system has been suggested to include the new rarer genetic diagnoses within the original four clinical types, rather than as separate disorders (Van Dijk & Sillence, 2014), or into five groups based on the underlying pathogenic mechanism (Forlino & Marini, 2016).
In addition to OI, mutations in COL1A1 can cause one form of Ehlers–Danlos syndrome (EDS) type I, EDS type VIIA, or Caffey disease (infantile cortical hyperostosis) (OMIM®, 2016). EDS type I is characterized by skin hyperextensibility, joint hypermobility, and easy bruising, and it is most commonly caused by heterozygous null mutations in COL5A1 (De Paepe & Malfait, 2012). However, EDS type I can rarely be caused by missense mutations in COL1A1 that substitute cysteine for non-glycine residues, typically arginine (R>C), in the triple-helix domain. EDS type VIIA, or arthrochalasis multiplex congenita, is caused by heterozygous mutations in COL1A1 that disrupt the N-terminal protease cleavage domain and prevent processing of proα1(I)to α1(I) (Byers et al., 1997). Patients with EDS type VIIA primarily demonstrate joint hypermobility and dislocation with less prominent skin or vascular involvement. Caffey disease is a very rare disorder characterized by excessive cortical bone formation, joint hypermobility, and skin hyperextensibility that has only been associated with the specific COL1A1 missense mutation p.R1014C and exhibits variable penetrance (Gensure et al., 2005; Nistala, Makitie, & Juppner, 2014).
Thus, genotype–phenotype correlations have been established for several different types of monoallelic COL1A1 mutations, although each resulting disorder has a spectrum of severity. Here we report a unique kindred of three siblings with biallelic mutations in COL1A1 that are associated with an atypical form of type I osteogenesis imperfecta.
2 MATERIALS AND METHODS
The index patient was referred to the Center for Bone Health at the Children's Hospital of Philadelphia (CHOP) for evaluation of a long bone fracture at a young age without significant trauma. Due to concern for OI, Sanger sequencing was performed for COL1A1 and COL1A2 coding exons. Once the two COL1A1 mutations were identified, her siblings and parents were also genotyped using Sanger sequencing of PCR-amplified exons 36 and 43. All sequencing was performed by the Connective Tissue Gene Tests laboratory (Allentown, PA). The family has refused skin biopsy for analysis of type I collagen in cultured skin fibroblasts. Mutations are denoted based on standard genome annotation, with the ATG start codon defining the +1 amino acid position within the protein (“p.”), and the A base of that codon representing the +1 position of the coding sequence of the transcript (“c.”). The study was approved by the Institutional Review Board of the Children's Hospital of Philadelphia, and informed consent/assent was obtained from all subjects.
3 RESULTS
3.1 Molecular genetics
All three children have compound heterozygosity for COL1A1 mutations, with a novel frameshift mutation (c.2522delC; p.Pro841Leufs*266) inherited from their affected mother and a previously reported (Cabral et al., 2007) missense mutation (c.3196C>T; p.R1066C) from their clinically unaffected father (Figure 1a).
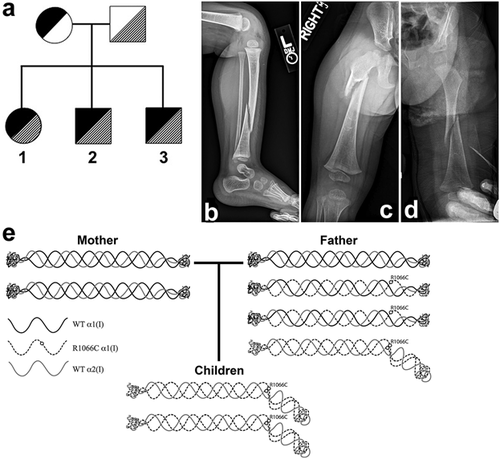
The maternal mutation, a novel c.2522delC; p.Pro841Leufs*266 mutation in COL1A1 (Dalgleish, 2015), results in a frameshift that changes the amino acid sequence beginning at codon p.841, which disrupts the canonical Gly-Xaa-Yaa triple amino acid repeat that is essential for the triple helical structure of the α1(I) protein (Forlino & Marini, 2016) and causes a premature termination codon 266 amino acids later at position p.1107 (exon 47) instead of p.1465 (exon 51). Typically, premature termination codons result in nonsense-mediated decay of the truncated mRNA, although some transcripts may escape this process (Holbrook, Neu-Yilik, Hentze, & Kulozik, 2004). However, escape from nonsense-mediated decay has not been described for COL1A1 mRNA with premature termination codons within the first 49 exons (Slayton, Deschenes, & Willing, 2000; Symoens et al., 2014; Willing, Deschenes, Slayton, & Roberts, 1996). Furthermore, because assembly of proα1(I) and proα2(I) chains into the procollagen tripeptide proceeds from the C-terminus to the N-terminus and requires specific C-terminal sequences (Forlino & Marini, 2016), any protein produced by the c.2522delC; p.Pro841Leufs*266 mutation that eliminates the C-terminus would not be incorporated.
The paternal COL1A1 mutation (c.3196C>T; p.R1066C) alters the Y position of a single Gly-Xaa-Yaa triple amino acid repeat in the triple helical domain, thus altering the structure of the α1(I) chain and resulting type I collagen molecule and fibril (Cabral et al., 2007). Based on what is known about COL1A1 genotype–phenotype correlations, this mutation would be expected to cause an EDS-like phenotype (De Paepe & Malfait, 2012). Indeed, this heterozygous mutation was previously reported in a pedigree of four family members as the cause of a mixed type I OI/EDS phenotype with moderately reduced bone mineral density (BMD; Z-score of −1.3 to −2.6), long bone fractures beginning in adolescence, mildly blue sclerae, and large joint hyperextensibility (Beighton score 2–4) (Cabral et al., 2007).
3.2 Mother
The heterozygous mother has a history of low BMD as determined by dual-energy x-ray absorptiometry (DXA) with lumbar spine L2-L4 Z-score of −3.8 (not height-adjusted) at 9 years of age and three long bone fractures that all occurred prior to puberty (Table 1). She was treated with a bisphosphonate (alendronate) from age 10 to 12 years, with subsequent improvement in BMD (Z-score −1.8, not height adjusted) and no further fractures after menarche. She has no bone deformities and has a normal height. She refused repeat DXA examination.
| Father | Mother | Sibling 1 | Sibling 2 | Sibling 3 | |
|---|---|---|---|---|---|
| Genotype | c.3196C>T | c.2522delC | c.2522delC; c.3196C>T | c.2522delC; c.3196C>T | c.2522delC; c.3196C>T |
| Current age | 29 y | 26 y | 4 y 9 m | 3y 7 m | 2 y 2 m |
| Current height SDa | 0.5 | 0.3 | −0.65 | −0.89 | −0.82 |
| Current height SDa—MPH SD | nd | nd | −1.05 | −0.89 | −0.82 |
| Fractures, age | None | Femur, | R fibula 17 m | R femur 21 m | L femur 12 m |
| Tibia, | L tibia 23 m | R tibia 24 m | L tibia 19 m | ||
| Tibia 9 y | R tibia 30 m | R ulna 31 m | L tibia 22 m | ||
| L calcaneus 40 m | R tibia/fibula 24 m | ||||
| L tibia 41 m | |||||
| L 1st metatarsal 44 m | |||||
| Serum calcium (mg/dl) | nd | nd | 10.5 | 10.0 | 10.3 |
| Serum phosphorus (mg/dl) | nd | nd | 5.2 | 6.4 | 5.7 |
| Serum alkaline phosphatase (U/L) | nd | nd | 227 | 227 | 256 |
| Serum PTH (pg/ml) | nd | nd | 17.4 | 11.4 | 14.3 |
| Serum 25-OH Vitamin D (ng/ml) | nd | nd | 34.9 | 22.5 | 11.5 |
| Lumbar spine BMD (g/cm2, Z-score, age) | 1.069, −0.2, 28 y | 0.348, −3.8, 9 y 0.748, −1.8, 12 y | 0.365, −2.6, 3 y 0.419, −0.9, 4 y | 0.365, −1.9, 33 m | nd |
| Joint mobility | Normal | Normal | Very hypermobile | Moderately hypermobileb | Normal |
| Skin extensibility | Normal | Normal | Normal | Normal | Normal |
| Echocardiogram | Normal | nd | nd | nd | nd |
| Sclerae | Normal | Light blue | Light blue | Light blue | Light blue |
| Scoliosis | None | None | None | None | None |
| Easy bruising | None | None | None | None | None |
| Facies | Normal | Triangular | Triangular | Triangular | Triangular |
| Dentition | Normal | Normal | Normal | Normal | Normal |
- y, year; m, month; SD, Z-score standard deviation; MPH, mid-parental height; nd, no data; R, right; L, left; PTH, parathyroid hormone.
- a Based on 2000 CDC growth chart.
- b Beighton score of 4. All laboratory data collected prior to initiation of zoledronic acid therapy.
3.3 Father
The father has no history of bone fractures and is very active. His height and weight are normal. This subject has no clinical features of OI or EDS (Table 1) that were previously described in members of the unrelated kindred who carried the same heterozygous c.3196C>T; p.R1066C mutation (Cabral et al., 2007). His BMD is normal, and he had a normal echocardiogram (Table 1).
3.4 Sibling 1
The proband was born at term and is now a 4-year-old female with notable large joint hypermobility who has had four long bone fractures with minimal trauma (Table 1). Height (96.3 cm) and weight (13.2 kg) are normal. Her first fracture (right fibula) occurred at 17 months of age, her second fracture (left tibia distal diaphysis) was at 23 months of age, and her third fracture (right tibia distal diaphysis) was at 30 months of age. X-rays at the time of her second fracture showed bone demineralization and growth arrest lines (Figure 1b), and she had low BMD on DXA with lumbar spine Z-score of −2.6 (not height Z-adjusted) at 35 months of age (Kalkwarf, Zemel, Yolton, & Heubi, 2013). Mineral metabolism studies were normal. Because of her low BMD and history of multiple fractures at a young age, treatment with the bisphosphonate zoledronic acid was started (George et al., 2015). She received intravenous (IV) infusions of 0.0125 mg/kg at 36 months of age and 0.025 mg/kg at 43 and 56 months of age. She has had one long bone fracture (left tibia distal diaphysis re-fracture) since starting treatment, which occurred 4 months after her first infusion of zoledronic acid and prior to her second infusion. Notably, her BMD Z-score at 48 months of age improved to −0.9 (not height Z-adjusted) after receiving two infusions of zoledronic acid. Her development has been normal, although her most recent height Z-score (−0.65) is below her mid-parental height Z-score (0.4).
3.5 Sibling 2
The second sibling was born at term and is now a 3-year-old male with mild joint hypermobility and a history of three long bone fractures with minimal trauma (Table 1). Mineral metabolism studies were normal. Growth and development have been normal, although his most recent height Z-score (−0.89) is below his mid-parental height Z-score (0.0). His first fracture (right femur diaphysis) occurred at 21 months of age. Because of the genotypic and phenotypic similarities with his older sibling, bisphosphonate therapy was initiated. He received IV infusions of zoledronic acid 0.0125 mg/kg at 22 months of age and 0.025 mg/kg at 29 and 41 months of age. His second fracture (right tibia diaphysis) occurred 1 month after his first infusion of zoledronic acid and his third fracture (right ulna diaphysis) occurred 2 months after his second infusion. Due to young age, he did not have his BMD evaluated by DXA prior to administration of zoledronic acid, but he had evidence of bone demineralization on radiographs (Figure 1c). His BMD Z-score at 33 months of age, after receiving 2 infusions of zoledronic acid, was −1.9 SD (not height Z-adjusted).
3.6 Sibling 3
The third sibling was born at term and is now a 2-year-old male with multiple long bone fractures. His development and biochemical studies of mineral metabolism were normal (Table 1). Similar to his siblings, his most recent height Z-score (−0.82) is below his mid-parental height Z-score (0.0). He was empirically started on bisphosphonate therapy based on his genotype and his siblings’ histories, although he had no fractures at the time. He received his first IV infusion of zoledronic acid 0.0125 mg/kg at 12 months of age and had his first long bone fracture (left femur diaphysis) 10 days later with minimal trauma. He had a second fracture (left femur metaphysis) with minimal trauma at 19 months of age, and then re-fractured that site at 22 months of age. His fourth fracture (right tibia/fibula) occurred at 24 months of age. He received his second dose of zoledronic acid (0.025 mg/kg) 2 weeks later. X-rays at the time of his first fracture showed bone demineralization (Figure 1d). He has not yet had a DXA scan. He has no clinical evidence of joint hypermobility.
4 DISCUSSION
This kindred provides a unique opportunity to assess the relative contributions and interactions of two different pathogenic COL1A1 mutations. The father in our kindred carries the c.3196C>T; p.R1066C missense mutation that was previously reported to cause a type I OI/EDS hybrid phenotype, associated with abnormal type I collagen structure, and stability (Cabral et al., 2007). This mutation replaces arginine with cysteine in the Y position of a single Gly-Xaa-Yaa triple amino acid repeat in the triple helical domain of proα1(I). Importantly, cysteine is normally specifically excluded from the triple helical domain; the presence of cysteine allows a disulfide bond to form between the mutant proα1(I) chains, disrupting the normal type I procollagen structure (Figure 1e). Indeed, the presence of disulfide bonds has been shown for the c.3196C>T; p.R1066C mutation (Cabral et al., 2007) described here, as well as for the p.R1014C mutation that causes Caffey disease (Gensure et al., 2005) and for glycine-to-cysteine substitutions (Lightfoot et al., 1992). Further support for the pathologic nature of the c.3196C>T; p.R1066C mutation includes its rare allele frequency of 1:22,071 in the European non-Finnish population based on the ExAC database (Lek et al., 2016), the p.R1066 codon's high conservation across species, and the results of in silico analyses using both SIFT and Polyphen-2 (score 1.0) which predict that the mutation is damaging. Although the father has no clinical phenotype, we cannot exclude the possibility that his mutation is present in a mosaic fashion in his osteoblasts, although there was no evidence of mosaicism in his peripheral blood leukocytes on review of his Sanger sequencing results (data not shown). Importantly, all other known carriers of the c.3196C>T; p.R1066C mutation have shown phenotypic features, with at least osteopenic BMD on DXA ([Cabral et al., 2007] and personal communication, Dr. Joan C. Marini, 2016). Moreover, this mutation does appear to affect all three of his compound heterozygous children, who have a more severe phenotype than either the four subjects who were heterozygous for the identical c.3196C>T; p.R1066C mutation (Cabral et al., 2007) or their mother who is heterozygous for their null COL1A1 mutation. This distinction in severity is based on the facts that the siblings described here had more fractures at younger ages and more significantly diminished vertical growth. These findings are consistent with the children having a reduced amount of type I collagen that is exclusively composed of two mutant α1(I) chains and one wild-type α2(I) chain, resulting in structurally abnormal type I collagen molecules and causing a combined type I OI/EDS phenotype. In contrast, their father has a normal amount of type I collagen with one-fourth of the trimeric molecules having abnormal structure similar to the children's molecules, one-fourth being wild-type, and one-half incorporating both wild-type and mutant α1(I) chains, while their mother has a reduced amount of exclusively wild-type type I collagen (Figure 1e).
To our knowledge, the three affected children described here are the first surviving subjects to be identified who carry biallelic pathogenic mutations in COL1A1. Although a child with inherited biallelic COL1A1 mutations was recently reported (Ju et al., 2016), only one of the mutations is definitely pathogenic (c.3235G>A, p.G1079S) and inherited from an affected parent, while the second mutation (c.3247G>A, p.A1083T) is not predicted to be damaging by in silico analyses or what is known about genotype–phenotype correlations of COL1A1 mutations and is inherited from an unaffected parent. Biallelic mutations in COL1A2 have been described in several patients, with a broad phenotypic spectrum ranging from mild EDS to severe OI (De Paepe, Nuytinck, Raes, & Fryns, 1997; Malfait et al., 2006). A patient with two different mutations resulting in glycine substitutions on the same COL1A2 allele, resulting in a severe OI phenotype, has also been reported (Takagi et al., 2015).
Given the significant number of long bone fractures with decreased BMD observed in the oldest siblings, we initiated bisphosphonate therapy in all three patients after the COL1A1 mutations were identified. Although controversial, this approach is supported at least in part by recent long-term follow-up studies that revealed significantly increased BMD for children with OI types III or IV who were started on IV bisphosphonate therapy before 5 years of age and continued therapy for at least 6 years (Palomo et al., 2015). However, the results were less encouraging for decreasing long bone fractures or improving vertical growth. This may be due to the lack of improved bone quality, despite increased measured bone density, in patients with non-type I OI who have defective collagen matrix. Preliminarily, it seems that bisphosphonate therapy has increased BMD in sibling 1, although whether this effect will be observed in the other siblings, whether it will persist, and whether it will decrease fracture rate are yet to be determined. Of note, the subjects described in (Cabral et al., 2007) were not treated with bisphosphonates (personal communication, Dr. Joan C. Marini, 2016).
In summary, we describe three siblings with compound heterozygous mutations in COL1A1, one of which is novel, that result in significant features of OI including low bone density and multiple long bone fragility fractures at a young age, as well as characteristics of EDS including large joint hyperextensibility. This is the first report of biallelic pathogenic COL1A1 mutations that result in constitutive incorporation of defective α1(I) chains into type I collagen molecules. Because the long-term outcomes of these mutations and the effects of bisphosphonate therapy in this population are unclear, it seems warranted to be cautious in the care of these patients.
ACKNOWLEDGMENTS
We thank the patients and their families for their contributions to these findings. We thank the clinical staff at the Center for Bone Health at the Children's Hospital of Philadelphia for their contributions to patient care. We appreciate discussions with Dr. Joan C. Marini. AMA is supported by a grant from the Juvenile Diabetes Research Foundation (3-PDF-2014-186-A-N).
AUTHORS’ CONTRIBUTIONS
MAL: Study design. AMA and MAL: Data collection, analysis, and interpretation. AMA: Drafting manuscript. AMA and MAL: Revising manuscript content. AMA and MAL: Approving final version of manuscript. AMA: takes responsibility for the integrity of the data analysis.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.