A case of familial transmission of the newly described DNMT3A-Overgrowth Syndrome
Abstract
DNMT3A-Overgrowth Syndrome (also known as Tatton–Brown–Rahman Syndrome) (MIM 615879) has recently been described in 13 individuals with de novo heterozygous mutations in DNMT3A gene. This autosomal dominant condition is characterized by overgrowth, dysmorphic facial features and moderate intellectual disability. Missense and truncating point mutations, a small in-frame deletion, as well as microdeletion 2p23 have been reported. Moreover, DNMT3A is commonly somatically mutated in acute myeloid leukemia. We herein report a family with two siblings and their father affected by the syndrome. The proband is a 12 year-old boy with tall stature, macrocephaly, facial dysmorphism, and intellectual disability. His 10-year-old sister also has learning difficulties, overgrowth and mild facial dysmorphism. Their father is a 49 year-old man with tall stature, macrocephaly, learning difficulties, and minor facial dysmorphism. He had a right occipital osteoma removed at 20 years of age. A heterozygous splice site mutation NM_022552.4 (DNMT3A): c.2323-2A > T was found in the proband by whole exome sequencing analysis and by targeted Sanger Sequencing for the proband's sister and father. This mutation has not been previously reported and is believed to be pathogenic. Indeed, this substitution involves a highly conserved canonical splice site and is predicted to cause exon skipping. This is the first report of a familial transmission of DNMT3A-Overgrowth Syndrome, supporting the autosomal dominant inheritance. The proband's phenotype is more severe than that of his two other affected family members, which illustrates variable expressivity in the syndrome.
1 INTRODUCTION
DNMT3A-Overgrowth Syndrome (also known as Tatton–Brown–Rahman Syndrome) (MIM 615879) has recently been described in 13 individuals with de novo heterozygous mutations in DNMT3A gene (Tatton-Brown et al., 2014 ). DNA methyltransferase 3A is involved in the formation and maintenance of genome-wide DNA methylation (Berdasco and Esteller, 2013 ; Walton et al., 2011 ).
This autosomal dominant condition is characterized by overgrowth, dysmorphic facial features and intellectual disability. Patients have tall stature (mean of +3 SD) and macrocephaly (mean of +2.5 SD). Intellectual disability is described as moderate in most patients. Facial characteristics include round face, heavy horizontal eyebrows, and narrow palpebral fissures. For the 13 reported cases, the clinical features that were observed more than once include scoliosis, seizures, umbilical hernia, and congenital heart defects.
Out of the 13 reported patients, 10 were found to have heterozygous missense mutations, two had truncating point mutations, and one had a small in-frame deletion (Tatton-Brown et al., 2014 ). Okamoto, Toribe, Shimojima, and Yamamoto (2016 ) reported a patient with a microdeletion that includes the DNMT3A gene. This patient had a phenotype compatible with DNMT3A-Overgrowth Syndrome, making haploinsufficiency a plausible mechanism. Also, DNMT3A is commonly somatically mutated in acute myeloid leukemia (Ley et al., 2010 ). We herein report a family with a father and his two children carrying a heterozygous mutation in DNMT3A gene. Their clinical features are consistent with DNMT3A-Overgrowth Syndrome.
2 CLINICAL REPORT
The proband is a 12 year-old boy with overgrowth, intellectual disability and facial dysmorphism. He was born by vaginal delivery at 40 weeks of gestation from a 28 year-old primiparous women after an uncomplicated pregnancy. His birth weight was 4.2 kg (97th centile), his height was 55 cm (97th centile) and his head circumference was 37 cm (97th centile). He had right congenital torticolis causing left positional plagiocephaly which resolved with physiotherapy. He had a dermoid cyst removed from his right forehead at 18 months of age. He presented with global developmental delay since early infancy. The most recent neuropsychological testing, performed at 12 years of age, showed mild intellectual disability. However, he has severe expressive and receptive language disorder with attention deficit disorder. No other behavioral anomalies were reported or observed. At 10 years of age, his height was 153.5 cm (+3.4 SD) with advanced bone age, his weight was 39.2 kg (+2 SD) and his head circumference was 56.5 cm (+2.2 SD). He has mild scoliosis and bilateral calcaneo valgus. Facial dysmorphisms include depressed nasal bridge, large forehead, long eyelashes, narrow palpebral fissures, thin upper lip, low anterior hairline insertion, and synophrys (Figure 1 A). His only medication includes lisdexamfetamine. He has no other known health issues. His abdominal ultrasound at 4 years of age was normal.

His father is a 49 year-old man with tall stature (206 cm [+5.25 SD]) and a head circumference of 61.3 cm (+3 SD). He had learning difficulties but was able to complete his high school education. He reports occasional anxiety, but no formal psychiatric diagnosis has been made. He currently works as a warehouseman. Minor facial dysmorphisms include broad nasal bridge, heavy horizontal eyebrows, thin upper lip, and large forehead (Figure 1 B). He had a right occipital osteoma removed at 20 years of age. He had an echocardiogram and electrocardiogram for symptoms of palpitations which were normal. He has no other known health issues and takes no medication.
The proband has a 10 year-old sister. This was the couple's second and only other pregnancy. Antenatal 20 week-ultrasound showed bilateral choroid plexus cysts. The pregnancy was otherwise uneventful. She was born by vaginal delivery at 40 weeks of gestation without any complications. Her birth weight was 3.9 kg (90th centile), her height was 53.5 cm (97th centile), and her head circumference was 37 cm (97th centile). She is in a regular 5th grade class but has learning difficulties requiring the help of a special educator. Her motor development was delayed. She has anxiety and socialisation difficulties compatible with autism spectrum disorder. She has attention deficit disorder with impulsivity treated with atomoxetine. At 10 years old, her height is 151.4 cm (+3 SD) with normal bone age, her weight is 36.6 kg (+1.5 SD), and her head circumference is 55.5 cm (+2.2 SD). Minor dysmorphisms include synophrys, low anterior hairline insertion, and whole body hirsutism (Figure 1 C). She has mild lumbar scoliosis and mild small joint hypermobility. An abdominal ultrasound at 2 years of age was normal.
3 METHODS
Genomic DNA was extracted from blood samples from the four family members. Exome sequencing was performed using SureSelect XT Clinical research Exome capture kit (Agilent genomics, Santa Clara, CA) followed by sequencing on a HiSeq2500 instrument (Illumina, San Diego, CA). Data processing, alignment (using a Burrows–Wheeler algorithm) and variant calling were done according to the Broad Institute Genome Analysis Tool Kit (GATK v4) (Broad Institute, ). Variant annotation was done using Annovar (Wang, Li, & Hakonarson, 2010 ). Rare variants were filtered against a custom gene list comprising 4,060 genes involved in inherited diseases.
Whole exome sequencing was performed on the proband. As per laboratory protocol, the likely candidate was validated by bidirectional Sanger sequencing in our clinical laboratory. Targeted DNMT3A mutation testing was then performed by Sanger Sequencing on the proband's sister and both parents in a clinical laboratory.
4 RESULTS
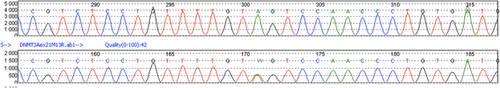
A heterozygous splice site mutation NM_022552.4 (DNMT3A): c.2323-2A > T was found in the proband, his sister, and their father (Figure 2 ). The mother was not found to carry the mutation.
5 DISCUSSION
The c.2323-2A > T splice site mutation in DNMT3A found in our three patients has not been previously reported. DNMT3A has three functional domains and the mutation involves the C-terminal DNA methyltransferase (MTase) domain. We believe that this mutation is pathogenic. Indeed, this substitution involves a highly conserved canonical splice site and is predicted to cause exon skipping. This mutation is found only once in the Exome Aggregation Consortium (ExAC) database (Exome Aggregation Consortium, ) and absent in the 1000 genomes database (European Bioinformatics Institute, ). Moreover, the patients’ phenotypes are consistent with DNMT3A-Overgrowth Syndrome.
We could not determine whether the mutation was inherited or arose de novo in the proband's father. His mother was of normal stature (168 cm) and no learning difficulties were known to the family. His father was 188 cm and worked as a manager. He died at 58 years of age from colon cancer. A paternal uncle of the proband is reported to be of tall stature (193 cm [+3 SD]); this individual's development is normal according to the family. The paternal grandfather of the proband had 16 siblings which were all reported to be of tall stature. Thus, other family members may possibly be affected. Extended familial testing in susceptible individuals is currently in progress.
DNA methyltransferase 3A is involved in the formation and maintenance of genome-wide DNA methylation (Berdasco and Esteller, 2013 ; Walton, Francastel, & Velasco, 2011 ). Our patient's mutation alters the functional domain of the DNMT3A protein and most likely interferes with histone binding and modifies the interaction with other DNA methyltransferases. This would result in reduced methyltransferase activity, thereby disrupting de novo DNA methylation, possibly through a loss-of-function mechanism. This hypothesis is supported by the recent report of a case of DNMT3A-Overgrowth Syndrome caused by DNMT3A deletion (Okamoto et al., 2016 ).
In general, abnormal de novo methylation of growth regulatory genes is associated with tumor development in humans (Baylin, Herman, Graff, Vertino, & Issa, 1998 ). Somatic mutations in DNMT3A gene are found frequently in hematologic malignancies (Ley et al., 2010 ). So far, no malignancies have been reported in patients with DNMT3A-Overgrowth Syndrome. The proband's 49 year-old father is the eldest reported patient with the syndrome to date. He only suffered from an osteoma, which is a benign skeletal tumor. DNMT3A has been reported to regulate osteoclast activity through epigenetic repression of anti-osteoclastogenic genes (Nishikawa et al., 2015 ). Hence, we suspect that it was involved in the pathogenesis of his bone tumor.
Also, the proband presented a dermoid cyst. The genetic basis of this type of tumor is not well studied. DNMT3A could be involved in its development through abnormal methylation of growth regulatory genes. We hereby report two benign tumors in patients with germline DNMT3A mutation. Further studies would be needed to determine the magnitude of the tumor risk in DNMT3A-Overgrowth Syndrome. It is not possible to determine with the available data whether these patients would benefit from a tumor surveillance strategy. Pending further study, we thus emphasize the need for clinical awareness regarding a possibly increased risk of benign or malignant tumors in DNMT3A-Overgrowth Syndrome.
Finally, this is the first report of a vertical transmission of DNMT3A-Overgrowth Syndrome from a father to his son and daughter. This is consistent with autosomal dominant inheritance. The proband's phenotype is more severe than that of his two other affected family members, which illustrates variable expressivity in the syndrome.
ACKNOWLEDGMENTS
We would like to thank the family for their cooperation and for allowing us to share their information.
Disclosures: The authors have no disclosures or other conflicts of interest to report.